, Patricia A. Noguera1 and Trygve T. Poppe2
(1)
Marine Scotland Science, Aberdeen, UK
(2)
Norwegian School of Veterinary Science, Oslo, Norway
Abstract
Necropsy is an essential part of an investigation of fish health of wild and farmed fish. Information on management practices and diet, detailed history of mortality, changes in fish behaviour, stock weight and length, management practices (recent or former transport, grading or treatments) as well as feeding response, are all important factors normally available with farmed fish. For wild fish, as much information as possible should also be collected and in both scenarios, water temperature, chemical and physical characteristics should be recorded with notes of any concurrent or recent event affecting other species in the area. The chapter describes the procedures of necropsy with particular reference to obtaining samples of the most common tissues collected for histological examination.
Keywords
Fish necropsy3.1 Introduction
Post-mortem examination or necropsy (from the Greek ‘nekros’: ‘corpse, dead’ and ‘opsis’: ‘eye’, ‘to see’), corresponds to the term autopsy when performed on the human body, namely the medical procedure of examining a body with the objective of assessing the cause of death and the lesions present. This is achieved through a systematic approach and observation of external and internal structures, organs or tissues, assisted by the collection of samples for further analysis. Necropsy plays a major role in the investigation of fish health of wild and farmed fish, both at the individual or the population level. Fish health assessment begins when the fish is alive in their habitat, when important observations can be made covering aspects related to clinical signs, the water and the environment. Under farming conditions, information on management practices and diet also become of particular relevance. Records of the number or the best estimate of affected individuals within the population should be ascertained to establish the morbidity rate and pattern of the spread of the disease or abnormality observed. If disease is associated with mortality, a detailed history of the daily and total mortality is required, taking into account age, class, and stock origin. Changes in fish behaviour including swimming pattern, position in the water column and respiratory patterns should be noted. Additional information on the stock average weight and length, management practices (recent or former transport, grading or treatments) as well as feeding response, are all important factors normally available with farmed fish. For wild fish, as much information as possible should also be collected and in both scenarios, water temperature and chemical and physical characteristics should be recorded with notes of any concurrent or recent event affecting other aquatic or terrestrial species in the area.
Compared to terrestrial animals there are limited laboratory tests applicable for live fish and therefore, generally clinical diagnosis is not enough for a conclusive diagnosis. This emphasises the need of the post-mortem examination as an essential step towards diagnosing disease in fish. The description provided in this chapter describes the procedures of necropsy with particular reference to obtaining adequate samples of the most common tissues collected for histological examination. This is on the understanding that during necropsy, other samples will also be taken e.g. for microbiological analysis, as well as blood or tissue samples for immunological, serological or molecular studies. However, these procedures will not be covered or discussed in detail in this book.
3.2 Sample Size and Euthanasia
The number of fish sampled for a health assessment will vary according to the objectives of the study. For example, certification of freedom of a notifiable disease generally follows the guidelines from the Office International des Epizooties (OIE). Here the sample size is based upon an assumed prevalence of the specific pathogen to an agreed level of confidence. To obtain a 95–98 % probability of detecting at least one infected fish in a clinically healthy population this translates to a minimum of 30 individuals. Conversely, for disease investigations 5–10 fish showing abnormal behaviour or the characteristic signs of the condition will be adequate for necropsy. Fish removed for examination should, where practical, be placed into a smaller container where further observations can be made before any procedure or the removal of tissues or body fluids. The fish should be euthanized by a humane method, ideally through an overdose of anaesthetic and ideally maintained at cool temperatures throughout the necropsy.
3.3 Necropsy Procedure
3.3.1 External Examination
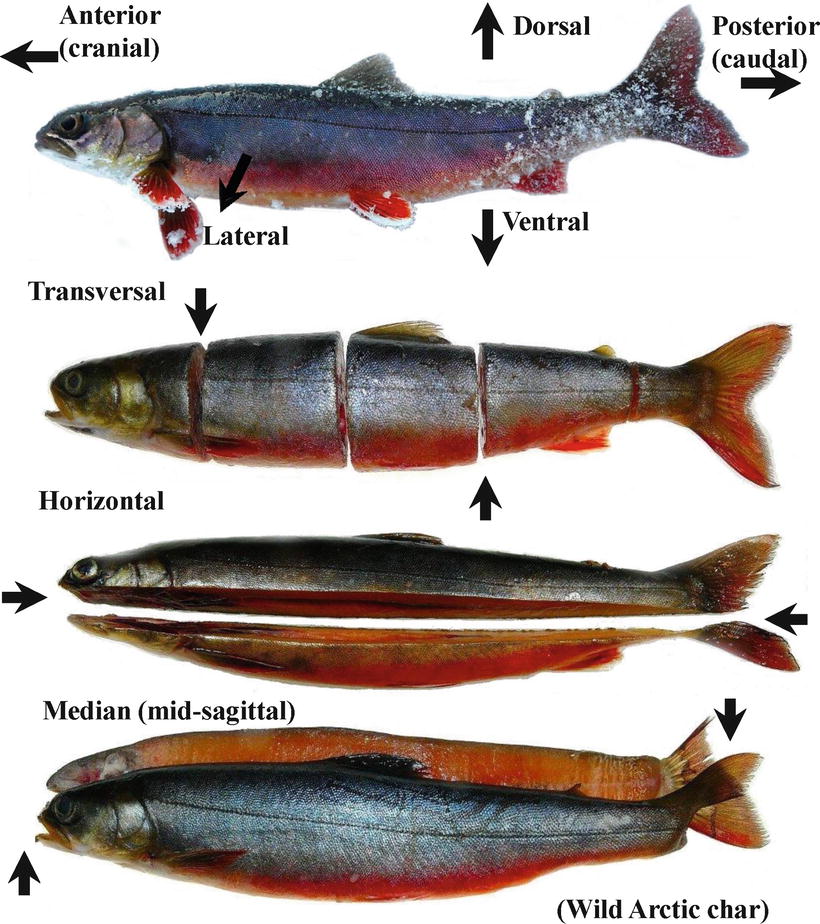
Fish should be placed on a surface that prevents further contamination to help the prosector to perform the work. A steel tray is ideal due to containment, easiness for disinfection and durability. Normally fish are placed on the right flank with head to the left, a convention based on fusiform fish such as salmonids, where internal organs become readily observable and easily accessed from the left flank, therefore requiring minimal displacement of organs to observe or access other structures. Different species and body forms (e.g. Pleuronectiformes) will require a different approach to achieve the same objective. The fish should be examined in a cool environment and case notes taken throughout the process of the post-mortem examination, and noting any deviation from normality for the species. The provision of a reference to the relative position of the abnormality or the sample taken is an essential part of the report; a few useful anatomical terms of location applicable to whole animals, tissues or histological sections are outlined in Fig. 3.1.