, Patricia A. Noguera1 and Trygve T. Poppe2
(1)
Marine Scotland Science, Aberdeen, UK
(2)
Norwegian School of Veterinary Science, Oslo, Norway
Abstract
Metazoa parasites are multicellular organisms where cells have differentiated into organised tissues and organs. These parasites may be found in all fish organ systems both in wild and in farmed salmonids, in fresh and sea water. Recently, parasites that would have been included as Protists are now considered metazoans, as the case of Myxozoa. The use of molecular tools are able to identify many Metazoan parasites and 12S ribosomal DNA primers have significant potential for metagenetic analysis, but should not detract or replace traditional histology to asses their impact on the host. In this book we have used the term infestation to represent ectoparasitic conditions and infection, as referring to those that are endoparasitic. This chapter will cover a selection of the many species of Metazoa that can be found in salmonids.
Keywords
MetazoaSalmonTroutMetazoa parasites are multicellular organisms where cells have differentiated into organised tissues and organs. These parasites occur in all organ systems of wild and farmed salmonids worldwide, in both fresh and sea water. Molecular methods are available for many Metazoaparasites and 12S ribosomal DNA primers have significant potential for metagenetic analysis. However, this should not detract or replace traditional histology. Recently, parasites that would have been included in Protist are now considered as Myxozoa and therefore they are covered in this chapter. We have used infestation to represent external ectoparasitic infestations and infection as referring to internal endoparasitic conditions. Where appropriate, Metazoa life cycles are summarised to improve understanding and the examples covered in this chapter are summarized in Table 9.1.
Table 9.1
Principal Metazoa parasites from salmonids
Name | Phyla or class | Common location | Environment |
|---|---|---|---|
Ceratomyxa shasta | Myxozoa | Intestine | FW |
Chloromyxum truttae | Myxozoa | Gall bladder | FW |
Kudoa thyrsites | Myxozoa | Musculature | SW |
Henneguya zschokkei | Myxozoa | Musculature | FW |
Myxidium truttae | Myxozoa | Liver | FW, SW |
Myxobolus cerebralis | Myxozoa | Cartilage, brain | FW |
Parvicapsula pseudobranchicola | Myxozoa | Kidney, pseudobranch | SW |
Sphaerospora truttae | Myxozoa | Kidney | FW |
Tetracapsuloides bryosalmonae | Myxozoa | Many organs | FW |
Philonema oncorhynchi | Nematoda | Abdominal cavity | FW |
Cystidicola farionis | Nematoda | Swim bladder | FW, SW |
Pseudoterranova decipiens | Nematoda | Musculature, liver | SW |
Eustrongyloides sp. | Nematoda | Abdominal cavity | FW |
Anisakis simplex | Nematoda | Musculature, abdominal cavity, vent | SWa |
Eubothrium spp. | Cestoda | Intestine | FW, SW |
Diphyllobothrium ditremum, D. dendriticum | Cestoda | On intestine, liver, abdominal cavity | FWa |
Sanguinicola spp. | Trematoda | Heart, gills | FW |
Cryptocotyle lingua | Trematoda | Gills, external surface | SW |
Diplostomum spathaceum | Trematoda | Eye, brain | FW |
Phyllodistomum umblae | Trematoda | Kidney, urinary bladder | FW |
Apatemon gracilis | Trematoda | Pericardial and abdominal cavity | FWa |
Cotylurus / Ichthyocotylurus spp. | Trematoda | Heart | FW |
Stephanostomum tenue | Trematoda | Heart | SW |
Nanophyetus salmincola | Trematoda | Many organs | FW |
Gyrodactylus salaris | Monogenea | External surface | FW |
Gyrodactyloides bychowskii | Monogenea | Gills | SW |
Discocotyle sagittata | Monogenea | Gills | FW, BW |
Acanthocephalus spp. | Acanthocephala | Intestine | FW, SW |
Echinorhynchus spp. | Acanthocephala | Intestine | Normally SWa |
Pomphorhynchus laevis | Acanthocephala | Intestine | FW |
Lepeophtheirus salmonis | Maxillopoda | External surface | SW |
Caligus elongatus | Maxillopoda | External surface | SW |
Caligus rogercresseyi | Maxillopoda | External surface | SW |
Argulus spp. | Maxillopoda | Skin, external surface | FW |
Salmincola spp. | Maxillopoda | Gills, opercula | FW, SW |
Margaritifera margaritifera | Bivalvia | Gills | FW |
Piscicola geometra | Annelida | Gills, external surface | FWa |
9.1 Myxozoa
9.1.1 Ceratomyxa shasta
Ceratomyxa shasta is a significant parasite of hatchery and wild juvenile anadromous salmonids on the Pacific coast of North America. Differences in susceptibility are reported between species of salmon as well as the range of responses to infection. Significant losses can occur in hatcheries, but C. shasta is also implicated as a primary cause of mortality among wild stocks. For example, the survival of Chinook salmon is reduced in locations where parasite densities are highest. Infection results in exophthalmia, lethargy and a dark body. Abdominal distension and haemorrhaging are common around the vent region with severe inflammation and necrosis of the intestine. Infection starts in the epithelium of the posterior intestine and progresses towards a multifocal inflammatory response with sloughing and mucosal necrosis. Histologically, an acute inflammatory reaction in the intestine can be observed with proliferation of the connective tissue of the intestinal caeca and massive infiltration by the developing trophozoites and other developmental stages with subsequent spread to other organs. The occlusion and destruction of the lumen is considered to be the cause of the mortality among infected fish. A limited granulomatous inflammation may develop in the viscera with consequential peritonitis.
The actinosporean stage of C. shasta develops in a polychaete, Manayunkia speciosa. Fish become infected by coming into contact with water containing the infective stage with subsequent migration from the gill epithelium into the gill blood vessels where replication and release of the parasite occurs. C. shasta spores are elongate and contain two polar capsules at the anterior margin (Fig. 9.1).
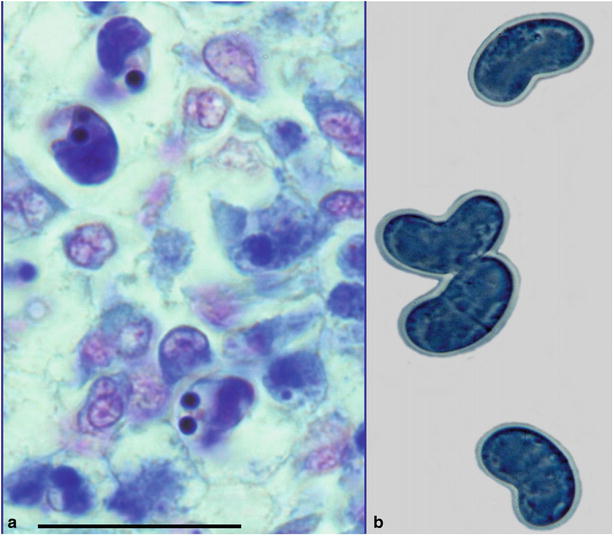

Fig. 9.1
Scrape showing characteristic elongate spores of Ceratomyxa shasta in steelhead trout. Bar = 20 μm. Giemsa stain (left). Fresh mount showing characteristic elongated crescent-shaped spores (right). High power
Diagnosis is carried out by the microscopic observation of typical spores in scrapes or stained sections prepared from the lower intestine, gall bladder or lesion within the body musculature. A polymerase chain reaction (PCR) is also available for diagnosis.
9.1.2 Chloromyxum spp.
Several myxosporeans are reported to infect salmonids, and C. truttae affecting farmed brown trout may result in a loss of appetite, emaciation and yellow discolouration of the skin and fins. Internally, the liver may be yellowish with hypertrophy of the gall bladder and associated enteritis. Infection can persist for several months and is fatal to some fish groups.
C. schurovi in Atlantic salmon and brown trout sporulates in the kidney tubules and reported that the vascular system is used for transport with proliferation occurring remotely in target tissues (Fig. 9.2).
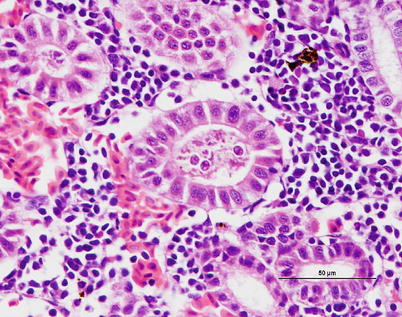

Fig. 9.2
Chloromyxum sp. plasmodia and spores in kidney tubules of brown trout. Medium power
C. wardi is reported from the gall bladder of chum salmon. Infection occurs in fry during the fresh water phase, but the formation of the spherical or oval spores does not take place until the fish returns to the marine environment.
Species determination is considered difficult without the use of scanning electron microscopy or PCR. However, phylogenetic relationships based on ribosomal DNA data of the 18S rDNA provides significant taxonomic detail although not always agreeing with traditional taxonomic classification.
9.1.3 Kudoa thyrsites
Kudoa thyrsites primarily affects the trunk muscle but can also be observed in the heart of many marine fish. In North America its broad host range include Atlantic, Chinook, coho and pink salmon and rainbow trout. Infection causes typical focal lesions which are often the cause of the myoliquefaction condition known as ‘milky flesh’.
Moribund fish are dark but generally no clinical signs are apparent until post-mortem. Internally, there is evidence of anaemia and the liver appears pale. The intramuscular stage starts with a single parasite in the muscle sacrolemma forming nodules or pseudocysts (Fig. 9.3). The plasmodium releases proteolytic enzymes which result in a histiolysis providing nutrients for growth of the plasmodia. Within the epicardium mononuclear cells infiltrate and an associated pericarditis may be evident. The dorsal musculature lesions show a characteristic multifocal intracellular infection with associated inflammatory response in the pericardium and myocardium. High numbers of Kudoa within the red and white muscle result in necrosis, fibrosis and inflammation followed by a chronic, active myositis with myolysis. In severely affected fish the kidney is swollen as a result of a markedly increased renal interstitium with occasional giant cells.
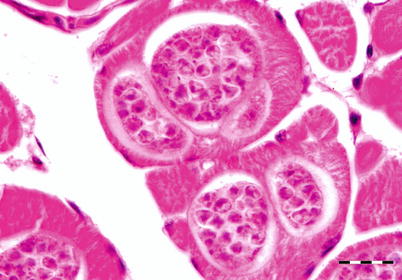

Fig. 9.3
Plasmodia of Kudoa thyrsites in transverse section of white muscle from Atlantic salmon. Bar = 20 μm
In Atlantic salmon a host response occurs after the polysporic plasmodia, containing the fully formed and the developing myxospores, rupture and the spores are released into the endomysium. The tissue damage and discolouration caused by Kudoa results in significant economic losses post-harvest as affected fish are rejected at processing.
Kudoa spores are stellate in shape and characterised by having four valves and four polar capsules, each containing a polar filament. Squash preparations, Gram or Giemsa stained imprints or sections of muscle tissue allows the spores to be observed.
9.1.4 Henneguya spp.
Henneguya zschokkei spores are found in the white muscle, cranial tissue and gills of different salmonids including Pacific salmon and whitefish from the Baltic Sea, while H. cartilaginis has been described from the head cartilage of wild masou salmon in Japan. Large, white-oval cysts with a creamy content occur and ruin the aesthetic appearance of the fillet (Fig. 9.4). The cysts mature and rupture through the integument and a large number of infective spores are discharged into the water. The open ulcers provide a port of entry for secondary pathogens. The diagnosis is based on the demonstration of the characteristic spores containing two polar capsules and two caudal projections.
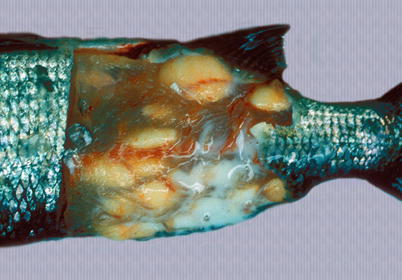

Fig. 9.4
Henneguya zchokkei cysts in white muscle of whitefish. Note punctured cyst with milk-like contents escaping
9.1.5 Myxidium spp.
Myxidium truttae is a common fresh water parasite particularly of the liver of wild and farmed salmonids in Eurasia. Myxidium salvelini has been reported in Arctic char, but is probably apathogenic. Grossly, affected livers may have pale or yellowish surface protrusions. On incision, a thick yellowish or creamy coloured fluid can be recorded (Fig. 9.5). Similarly, Myxidium minteri has been reported in kidney of coho salmon (Fig. 9.6).
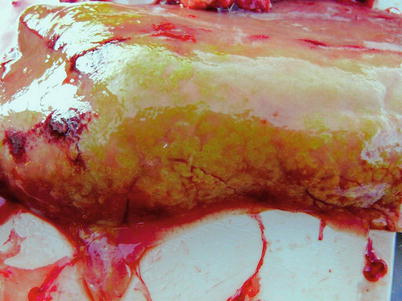
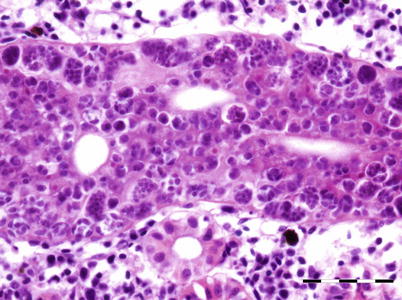

Fig. 9.5
Liver of adult wild Atlantic salmon with numerous plasmodia of Myxidium truttae protruding above the liver surface

Fig. 9.6
Myxidium minteri in kidney of coho salmon. Bar = 50 μm
During the life cycle, actinospores are discharged from an annelid host into the water and penetrate the skin of the fish intermediate host. The sporoplasm develops into presporogonic stages and eventually will locate in the bile ducts where the sporogonic stage, plasmodium develops. The cytoplasm of these large worm- or sac-like structures is filled with many cell types including sporogonic cells and pericytes. These develop into sporogony (spore formation), and subsequently into sporoblasts and myxospores.
Histological examination reveals dilated bile ducts and excretory canals with characteristic worm-like plasmodia filled with sporogonic cells and spores (Fig. 9.7). Diagnosis is based upon the examination of wet mounts or tissue sections, the identification of typical plasmodia and crescent shaped or fusiform spores and polar capsules at opposing ends.
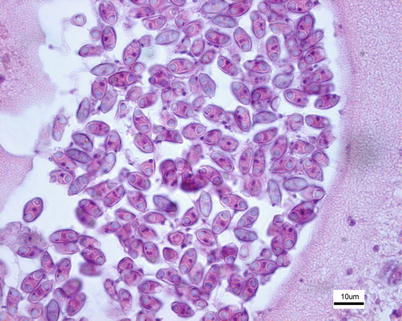

Fig. 9.7
Myxidium truttae spores in bile duct plasmodium from wild Atlantic salmon
9.1.6 Myxobolus cerebralis
Myxobolus cerebralis is the causal agent of a persistent and economical important condition termed ‘whirling disease’ (WD). The parasite has been identified from farmed and wild Pacific and Atlantic salmon, but within farmed species this is mainly a problem in trout reared in earthen ponds. Clinical signs include spiralling (‘whirling behaviour’), darkening of the caudal region and severe skeletal deformities of the cranial area, jaw and opercula (Figs. 9.8 and 9.9) The whirling behaviour is often pronounced when the fish attempt to feed or when the fish are startled. Erosion of the cartilage surrounding the auditory organ is reported to contribute to the whirling. Infection of the cartilage of the spinal column causes pressure on the caudal nerves resulting in loss of control of caudal dermal melanophores. Infections cause a significant pathology usually in the first 3–4 months after fish start feeding and before ossification is complete. Maturing stages lyse and digest chondrocytes thus disrupting osteogenesis. Thereafter, older fish may be infected but infections are unlikely to result in a clinical complication.



Fig. 9.8
Darkening of the caudal region in brown trout due to infection with Myxobolus cerebralis

Fig. 9.9
Skeletal deformity in brook trout following infection with Myxobolus cerebralis
Spores are released from the cartilage after death of the fish where they are ingested by an intermediate host, the oligochaete Tubifex tubiflex, in which they localize in the intestinal epithelium and develop into the triactinomyxon stage. Re-infection in the fish host may occur through the skin and buccal cavity. Trophozoites migrate through the epidermis or gut lining and peripheral nerves to reach the cranial cartilage. In rainbow trout successful development of the parasite only occurs in sac fry which are older than 2 days. Trophozoites induce necrosis either directly or indirectly and lysis in the cartilage of the head and vertebrae. As ossification proceeds, there is an interruption of osteogenesis resulting in permanent cranial and other skeletal deformities. Trophozoites may also enter the labyrinth, causing damage and subsequent behavioural changes such as loss of balance. Although some fry die before any signs of WD are recorded, in most cases the course of the disease is chronic and spores remain for several years.
The classification of the group has been reanalysed and currently phylogenomic analyses of new genomic sequences of Myxobolus cerebralis firmly place Myxozoa as sister group to Medusozoa within Cnidaria. The morphological features of the spore which develop from the small amoeba-like trophozoite following infection of the fish are typically oval, measuring 8 × 10 μm and the two polar capsules are normally of equal size measuring 3 × 4 μm (Fig. 9.10). The multinucleate trophozoite grows and divides by nuclear division producing pansporoblasts, each of which will produce two spores which localise in the cartilage. Spore development in the fish has been linked to their acquisition of acid-fastness, and such spores have been referred to as ‘mature’.
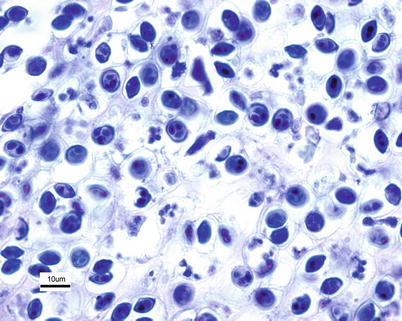

Fig. 9.10
Myxobolus cerebralis in cartilage of brook trout. Giemsa. High power
Diagnosis of whirling disease is made microscopically from stained tissue sections (e.g. Giemsa, Mallory-Heidenhain) of cartilaginous tissue or from wet mounts of macerated cartilage. Other species e.g. M. arcticus and M. neurobius infect the nervous tissue of salmonid species in fresh water in North America and Asia while M. insidiosus occurs in the striated musculature of Chinook salmon. Molecular genetic tests based on nuclear DNA are used to verify species.
9.1.7 Parvicapsula spp.
Parvicapsula pseudobranchicola is a serious pathogen in sea-water farmed Atlantic salmon in Norway, particularly in northern areas. It primarily affects the pseudobranch where it may cause extensive inflammation and necrosis with up to 40 % mortality, although other organs may also be affected. Affected fish show unspecific clinical signs that may include cataract, snout ulcers and increased numbers of ‘poor doing fish’. At autopsy, the pseudobranch is haemorrhagic or totally absent, leaving only a white pseudomembrane or a dark edge (Fig. 9.11). Histopathology reveals extensive infiltration of extrasporogonic stages, haemorrhage and necrosis of pseudobranchial tissue. The final host of this parasite are oligochaetes, while fish are the intermediate host. Infection occurs when actinospores are released from the final host and attach to the fish by means of the filaments from the polar capsules. The sporoplasm penetrates the epidermis and asexual reproduction follows, whereby new cells are infected. Mature spores are subsequently formed in the pseudobranch and are released into the environment allowing the final host infection. In this process, severe damage is inflicted on the pseudobranch resulting in considerable necrosis and loss of functional tissue. Parvicapsula can occur concurrently with other conditions and therefore the significance of the infection can be difficult to establish. Parvicapsula spores have a characteristic bean-or banana-shaped outline in smears from the affected pseudobranch (Fig. 9.12). Diagnosis is based upon gross, histological lesions and RT-PCR.
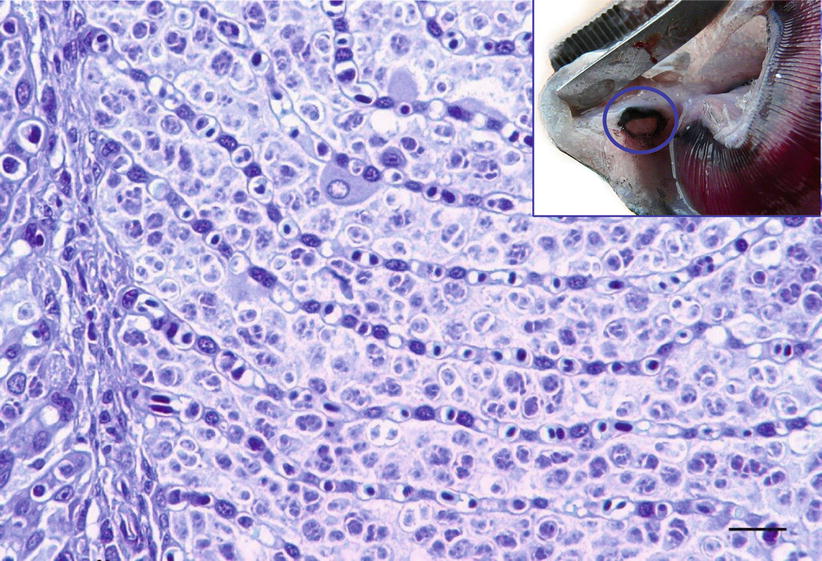
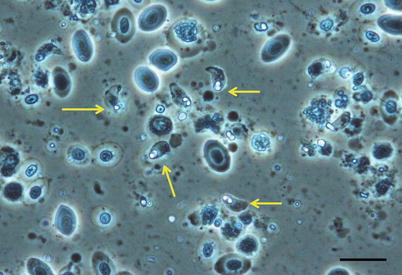

Fig. 9.11
Parvicapsula pseudobranchicola spores in pseudobranch of farmed Atlantic salmon. Low power. Insert Pseudobranch of farmed Atlantic salmon damaged by Parvicapsula pseudobranchicola

Fig. 9.12
Fresh squash mount from Atlantic salmon pseudobranch showing Parvicapsula pseudobranchicola spores (arrows). Dark field microscopy. High power
Parvicapsula minibicornis may infect several species of wild and farmed Pacific species and farmed Atlantic salmon in the northwest coast of the USA (Puget Sound, Washington region). Elevated pre-spawning mortality in some of these species has been associated to infection with P. minibicornis. Clinical signs and pathological changes are unspecific and may include dark and lethargic fish with hypertrophied kidney (Fig. 9.13). Histologically, trophozoites and developing spores occur in the lumen and epithelium. A PCR assay has identified the myxospore in the freshwater polychaete, Manayunkia speciosa.
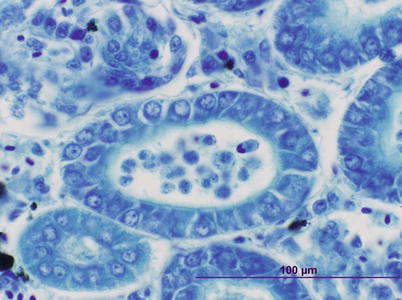

Fig. 9.13
Parvicapsula minibicornis spores in kidney tubules of juvenile coho salmon. Giemsa stain
Parvicapsula kabatai has been described from renal tubules of pink salmon in British Columbia, Canada. The shape and size of the spores are similar to those of P. pseudobranchicola, but distinctly different from P. minibicornis. The significance of this parasite is currently unknown.
9.1.8 Sphaerospora truttae
Sphaerospora truttae was originally described from brown trout and grayling in Germany, but subsequently reported affecting and Atlantic salmon parr in Scotland. Brown trout are also proven susceptible. The gills have been identified as the predominant point of entry, which is followed by penetration of the vascular epithelia and thereafter, proliferation in the blood before exiting the vascular system through capillary walls. Subsequently, the kidney, as well as the spleen and the liver are infected. Parasites occur in the tubular lumen and sporogony takes place inside the renal tubules (Fig. 9.14). Histology can be used for presumptive identification but for early myxosporean stages and parasite specific identity, a DNA-based approach is appropriate.
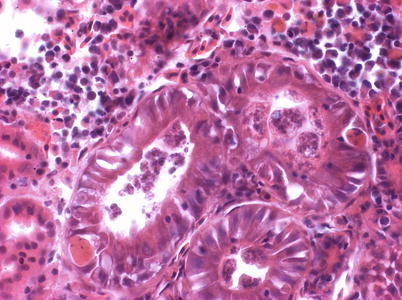

Fig. 9.14
Sporogonic stages of Sphaerospora truttae kidney tubules of farmed rainbow trout. High power
9.1.9 Tetracapsuloides bryosalmonae
Tetracapsuloides bryosalmonae causes the condition proliferative kidney disease (PKD) which is a significant seasonal disease of young salmonids. PKD occurs both in farmed and wild fish and is associated with decline in wild populations in many countries. The endoparasitic myxozoan uses freshwater bryozoans as primary hosts. Environmental changes may play a role in the increased significance of PKD in wild populations. Bryozoans and T. bryosalmonae stages in bryozoans undergo temperature and nutrient-driven proliferation and above 15 °C are required for development of clinical disease. Infective spores enter the fish through the skin and gill epithelium.
Clinical signs include a dark body, bilateral exophthalmia, pale gills, and distended abdomen with pale visceral organs. Swelling due to extensive accumulation of ascites is recorded within the abdomen. The kidney, particularly the caudal region, is markedly swollen due to diffuse oedema (Fig. 9.15) and to a lesser extent the spleen.

Fig. 9.15
Proliferative kidney disease in rainbow trout. Gross enlargement of the posterior kidney due to granulomatous response
Histozoic and extrasporogonic stages proliferate causing focal or multifocal granulomatous inflammation and renal interstitial tissue is replaced by mild haematopoietic hyperplasia during the early stages of infection, and followed by further granulomatous tissue with associated macrophages and mononuclear cells. Lymphoid cells and macrophages are frequently seen adherent to the PKX cells (Fig. 9.16) and hepatic lesions frequently include multinucleated giant cells scattered throughout (Fig. 9.17). There may also be extensive haemorrhage in the acute stages of the disease. The number of nephrons and melanomacrophage centres are severely reduced and an extensive chronic fibrosis occurs in the final stages.
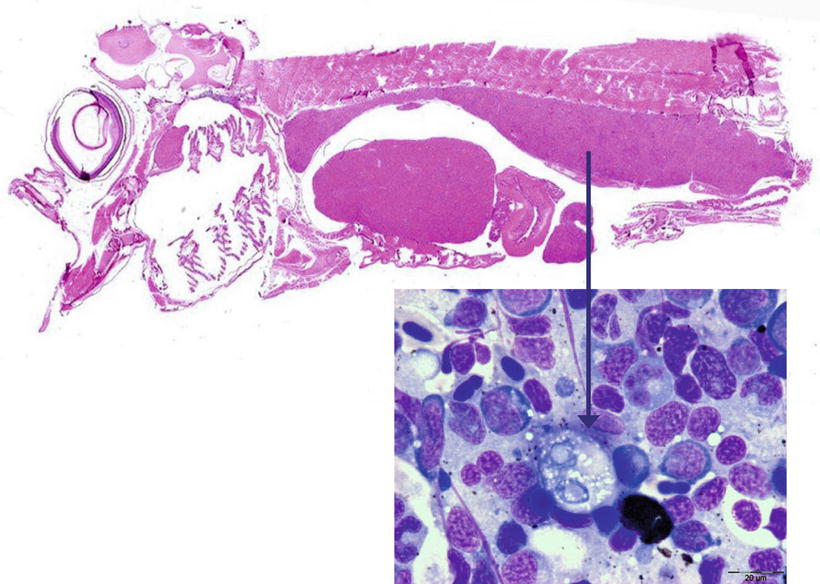
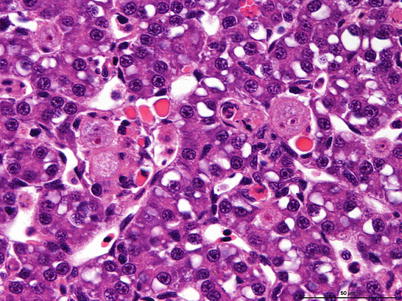

Fig. 9.16
Sagittal section of Atlantic salmon fry showing enlarged posterior kidney. Insert shows pre-sporogonic stage cell (arrowed). Insert Giemsa stained. High power

Fig. 9.17
Characteristic pre-sporogonic stage of Tetracapsuloides bryosalmonae in liver from Arctic char. High power
Throughout the affected tissue characteristic PKX cells can be found, particularly in the endothelium of the portal vessels. These cells are large, eosinophilic to pale orange (often multinucleated), PAS-positive cells with a granular cytoplasm and typically surrounded by a clear halo. PKX cells may be seen in several other organs including the gills, spleen, liver and heart. Developmental stages (sporoblasts) of the parasite may also be recorded in the walls and lumen of the kidney tubules.
The diagnosis of PKD is based on clinical signs and the demonstration of the characteristic extrasporogonic stages in wet preparations, stained imprints or in histological sections. In addition, a specific PCR assessment can be used as a confirming test. Sphaerospora truttae is important from the viewpoint of differential diagnostics.
9.2 Nematoda
The Nematoda comprise numerous species of unsegmented roundworms that possess a pseudocoelom and a threadlike body that is circular in cross-section. Nematodes in fish can be found both as larvae or adult endoparasites. Nematode infection causes a variable but generally not extensive pathology, although more prominent where the parasite is migrating or degenerating (Fig. 9.18). Nematode digestive tract is divided into mouth (buccal) capsule, oesophagus, intestine and rectum. Histologically, a multi-layered cuticle which can be ornamented with ridges is recorded. The hypodermis is often thinner than the somatic musculature often with extensions called lateral chords protruding into the pseudocoelom. The musculature is composed of dense contractile elements and pale sarcoplasm. In section the oesophagus is recognised by the radial symmetry and triradiate lumen. The structure of the epithelial cells range from large multinucleate cells to cuboidal or tall columnar cells and can be been used to differentiate groups.
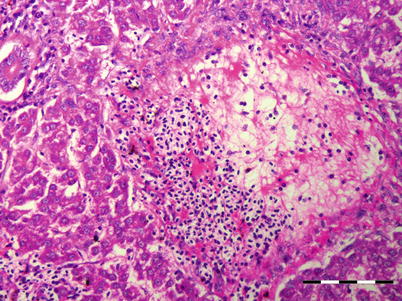

Fig. 9.18
Presumptive tract of migrating nematode in liver of Atlantic salmon. Low power
9.2.1 Philonema oncorhynchi
Philonema oncorhynchi (Philometridae) is noted in the visceral cavity of several fish species including rainbow trout, Arctic char, chum salmon and sockeye salmon in freshwater from various regions including Canada, Iceland, Japan and parts of Europe. P. sibirica is also described from European salmonids. Larva, sub-adult and adult worms can be present but are not usually fatal.
Juvenile fish acquire the nematode in freshwater by feeding on copepods, i.e. Cyclops spp. that act as the intermediate host. Larvae are released from gravid females present in the body cavity of affected hosts at spawning, and infect a copepod where they moult twice in their haemocoel into third stage larvae. The cycle is completed when the copepods are eaten by the final host. The orientation of seaward-migrating sockeye smolts is thought to be influenced by the presence of this parasite, which may have implications for smolt survival.
The parasite lives in the gastrointestinal tract migrating into the visceral cavity where it matures; it rarely migrates to the musculature but it may induce distension of the abdomen and ascites (Fig. 9.19). Activation of peritoneal macrophages, neutrophils and eosinophilic granular cells (EGCs) result in the formation of visceral adhesions, characterised by the production of fibrous connective tissue from the host preventing the organs from hanging freely in the coelomic cavity. In some severe cases adhesions may cause atrophy of the gonads and prevent spawning.


Fig. 9.19
Philonema salvelini in the peritoneal cavity of brook trout
Diagnosis of Philonema occurs at necropsy and is based upon the detection of a filiform worm up to 10 cm in length (female) with a rounded anterior end and a posterior tail tapering to a sharp point.
9.2.2 Cystidicola farionis
Cystidicola farionis is relatively common, particularly in wild salmonids, but also reported from farmed fish within Europe and North America. Adult males and females live in the swim bladder (Fig. 9.20) of fish including grayling, whitefish and rainbow trout. A pneumatic duct connected to the intestine is used by female worms to enter the intestine where eggs are deposited. Eggs are passed in the faeces and are eaten by amphipods, the first intermediate host. The L1 stage penetrates into the haemocoel and moults to the L3 stage, which is infective to the definitive host. After the infected intermediate hosts are consumed the L3 migrates up the pneumatic duct and completes its adult development.




