, Patricia A. Noguera1 and Trygve T. Poppe2
(1)
Marine Scotland Science, Aberdeen, UK
(2)
Norwegian School of Veterinary Science, Oslo, Norway
Abstract
Some viral diseases are prevented through vaccination but for many no vaccines are available hence infection is a major concern for farmed fish. Among salmonids, RNA viruses have the greatest ecological and socio-economic impact. Acute infections may lead to host death, or induce a more chronic condition. Stress including overcrowding, sexual maturation and handling may also reactivate a latent infection resulting in clinical disease. This chapter covers a range of viral diseases in wild and farmed salmonids.
Keywords
Fish virusSalmonTroutInfectious viral diseases continue to be a major concern in wild and farmed fish and responsible of important losses, particularly under farming conditions. These infections can manifest as a septicaemia-like disease with a characteristic acute presentation, but some viral agents may induce neoplasia or a more chronic condition. Overall, the consequences of viral infections depend on the outcome of the interaction between a number of factors both from the virus and the host. Acute infections may lead to host death, recovery with no long term effects, or develop into a chronic infection. The later can remain subclinical for life, have a long silent period before it manifests or can have periods of reactivation with relapses and exacerbation causing acute disease. Different types of stress including overcrowding, sexual maturation and handling may reactivate a latent infection resulting in clinical disease. Avoidance of viral infection is difficult, unless spring or disinfected water is used. This is possible in the early developmental fresh water stage, but very costly or impractical when farming in open environments e.g. sea water cage culture, or when high volumes are required for growers in land based systems.
Among salmonids, RNA viruses have the greatest ecological and socio-economic impact. The occurrence of viral diseases has seen an increment over the last 10 years and it is likely that more will be identified in the future. The most important conditions and host species are listed in Table 5.1.
Table 5.1
Principal viral diseases of salmonids
Virus name | Family | Nucleic acid | Principal salmonid host (s) | Environment |
|---|---|---|---|---|
Infectious pancreatic necrosis virus | Birnaviridae | ssRNA | Rainbow trout, salmon | FW, SW |
Infectious salmon anaemia virus | Orthomyxoviridae | ssRNA | Atlantic salmon | SW |
Oncorhynchus masou virus | Herpesviridae | ssRNA | Pacific salmon (e.g. masu) | FW? |
Piscine reovirus (Heart and skeletal muscle inflammation) | Reoviridae | dsRNA | Atlantic salmon | SW |
Salmon leukaemia virus | Retroviridae | ssRNA | Chinook salmon | SW, FW reared salmon in United States |
Viral haemorrhagic septicaemia virus | Rhabdoviridae | ssRNA | Rainbow trout | Mainly FW |
Infectious haematopoietic necrosis virus | Rhabdoviridae | ssRNA | Salmon, trout | FW |
Salmonid alphavirus | Togaviridae | ssRNA | Atlantic salmon, rainbow trout | FW, SW |
Piscine myocarditis virus (Cardiomyopathy syndrome) | Totiviridae | dsRNA | Atlantic salmon | SW |
Erythrocytic inclusion body syndrome | Iridovirus | dsDNA | Pacific, Atlantic salmon | FW, SW |
5.1 Infectious Pancreatic Necrosis
Infectious pancreatic necrosis virus (IPNV) is the aetiological agent of the highly contagious and acute catarrhal enteritis ‘infectious pancreatic necrosis’, primarily affecting cultured fish including rainbow trout and Atlantic salmon worldwide. The virus has a widespread distribution in many wild fish species but there is little evidence of transmission to farmed stock.
Significant mortalities can occur from clinical outbreaks in fry, with a relative reduced mortality in older fish. However, outbreaks in post-smolts in sea water are common and fish which survive infection can become carriers and continue to shed infective material into the water. Carrier fish are a particular hazard if used as broodstock since the virus has a germ-line associated vertical transmission via ova or milt, which occur both extra- or intra-ovum. Surface extra-ovum infection of the gametes can be managed through proper biosecurity procedures and disinfection, but the intra-ovum transmission imposes an important risk and can only be controlled through rigorous testing of the broodstock.
Salmonids may show clinical signs with high mortality or become asymptomatic carriers, thus establishing covert infections. Clinical signs include moribund fish which are darker in appearance, slightly emaciated and lethargic. Abdominal distension, mild to moderate exophthalmia, haemorrhage in ventral areas, oedema and swelling at the vent are typical macroscopic findings. The liver and spleen appear pale with the stomach and intestine devoid of food (Fig. 5.1). Some petechial haemorrhage, particularly on the peri-pancreatic fat among the pyloric caeca may be recorded and similar gross signs are reported in infected salmonids reared in sea water (Fig. 5.2).
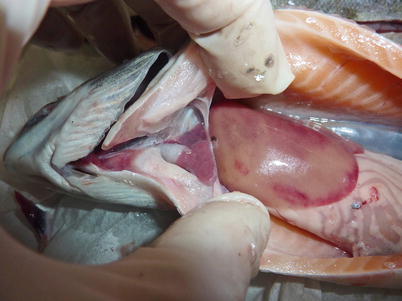
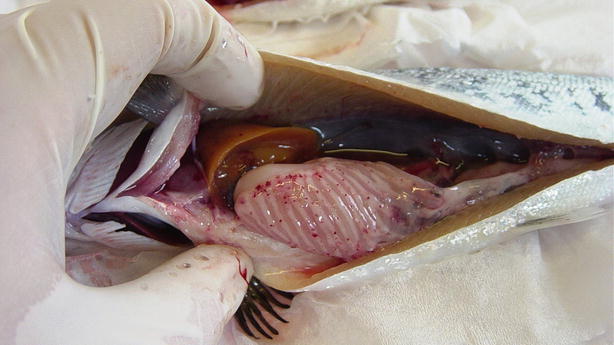

Fig. 5.1
Pale heart and liver with haemorrhage in farmed salmon post smolt with infectious pancreatic necrosis

Fig. 5.2
Petechiae in pancreatic tissue and peripancreatic fat in farmed salmon smolt with infectious pancreatic necrosis
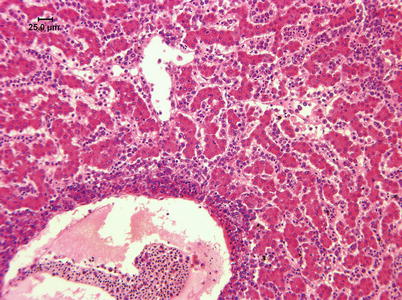
Histologically, IPN is a subacute to acute infection with pyknotic nuclei and associated focal necrosis of the pancreatic acinar tissue (Fig. 5.3). Areas of focal necrosis are replaced by a loose fibrous network with fat degeneration and consequently the tissue has a loose appearance in stained sections. In the gut, some apoptotic cells are noted within the mucosal intestinal epithelium (‘McKnight’ cells),with sloughing combined with excess mucous to form a haemorrhagic exudate (Fig. 5.4). An increase in eosinophilic granular cells in the granulosum layer of the intestinal wall can be recorded. Pancreatic and hepatic tissues may be infiltrated by macrophages and polymorphonuclear leucocytes. Involvement of the liver has been documented as a feature of IPN, with early changes described as fine vacuolation or vesicles within individual hepatocytes. Progressively the vacuolation becomes widespread, particularly towards the external edge of the hepatic cords and individual lobules, and mirrored by an increase in apoptotic figures (Fig. 5.5). Apoptotic bodies are phagocytised by neighbouring cells with only a mild or generally absent inflammatory response (Fig. 5.6). Post apoptotic necrosis is observed in severely damaged livers, but extensive apoptosis can also take place without necrosis. Pyknotic nuclei with loss of cell integrity are found concurrently with the occurrence of apoptosis, becoming a prominent feature in advanced stages (Fig. 5.7). Occasionally the entire tissue becomes affected and the number of cells undergoing necrosis can be significant and outnumber those cells that are specifically apoptotic. Differential diagnosis of IPN includes other viral infections that can occur concurrently, such as salmonid alphavirus in Atlantic salmon post-smolts.
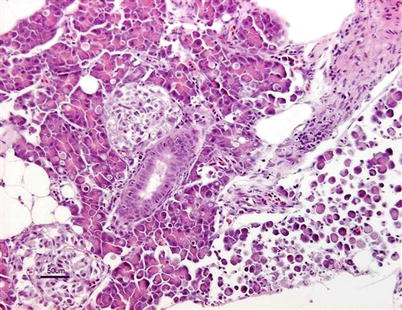
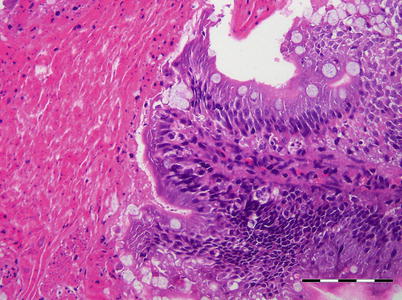
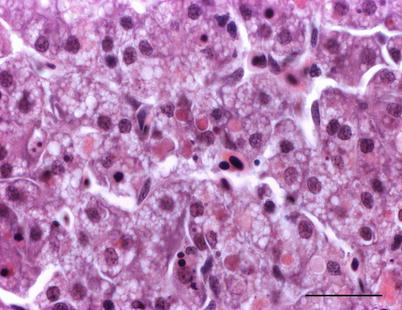
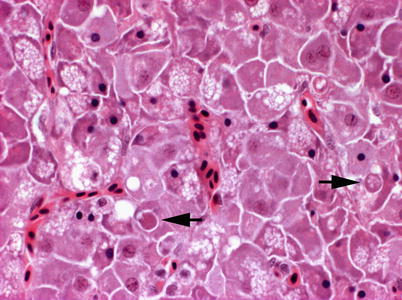
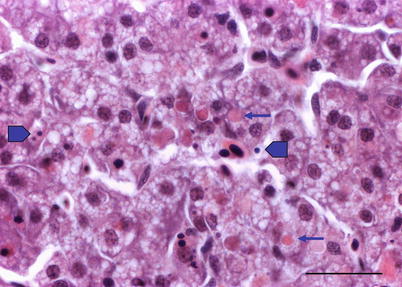

Fig. 5.3
Infectious pancreatic necrosis in farmed Atlantic salmon smolt. Necrosis of exocrine pancreatic cells (lower right) and normal tissue (upper left)

Fig. 5.4
Exudate in the intestine of farmed Atlantic salmon with infectious pancreatic necrosis. Bar = 100 μm

Fig. 5.5
Fine vacuolation or vesicles within individual hepatocytes in Atlantic salmon with infectious pancreatic necrosis. Medium power

Fig. 5.6
Liver of Atlantic salmon infected with infectious pancreatic necrosis showing apoptosis (arrows). High power

Fig. 5.7
Liver of Atlantic salmon infected with infectious pancreatic necrosis. Pyknotic nuclei (block arrows) with loss of cell integrity are found concurrently with apoptosis (arrows) and necrosis. Bar = 20 μm
Both clinical signs and histopathology are used to provide a presumptive diagnosis. A confirmed diagnosis of IPN can be achieved through tissue culture using a variety of salmonid and non-salmonid cell lines including CHSE-214 (Chinook salmon embryo), BF-2 (bluegill fry) RTG-2 (rainbow trout gonad), a neutralisation test, or a reverse transcription-polymerase chain reaction (RT-PCR) and sequence analysis. In addition, the detection in adherent leucocytes using immunofluorescence labelled specific antibodies is useful (Fig. 5.8). Infectious pancreatic necrosis virus is a bisegmented double-stranded RNA virus of the family Birnaviridae and at least nine serotypes exist. Commercially, both oral and injectable vaccines are available.
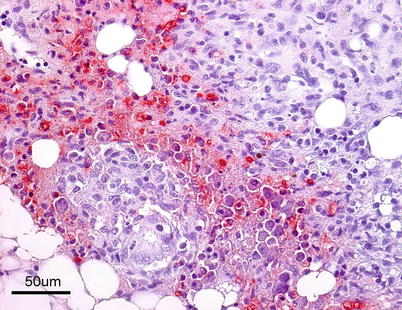

Fig. 5.8
Farmed Atlantic salmon with infectious pancreatic necrosis. Strong positive immunohistochemical reaction in post-vaccination granulomatous tissue in pancreatic area
5.2 Infectious Salmon Anaemia
Infectious salmon anaemia (ISA) is a highly infectious viral disease which has only been observed naturally in Atlantic salmon. ISA was first diagnosed in Norwegian aquaculture in the mid 1980s, but also reported from farmed fish in Canada, Faroe Islands, Scotland, USA, Ireland and Chile. Outbreaks of ISA occur in sea water or in hatcheries where sea water is mixed with fresh water for pH or adjustment to enhance smoltification. Under experimental conditions, sea-run brown trout can harbour and transmit the virus. Most new outbreaks have occurred in connection with rapid changes in temperature during the spring and to some extent in the autumn. Importantly, salmon stocks show large variation in their susceptibility to the virus.
Diseased fish are lethargic and listless and tend to sink to the bottom of the cages. During the acute stage, mortality is usually high but in the pre-acute stages mortality may not be observed. Externally, fish show abdominal distension, haemorrhagic spots surrounding the lens, pale gills and petechiae. Occasionally, some fish are observed with hyperactive behaviour and presumably nervous movements, spinning around the longitudinal axis. Internally, ascites, oedema of the swim bladder, splenomegaly and a homogeneous dark liver are common (Fig. 5.9).
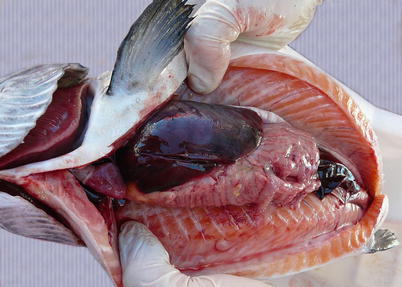

Fig. 5.9
Characteristic dark liver in Atlantic salmon with infectious salmon anaemia
The virus is described as non-cytolytic endotheliotropic and infection of the endothelium lining the circulatory system is observed without vasculitis and with virus absent from necrotic parenchymal cells, e.g. in liver, heart or kidney. The pattern of virus attachment mirrors the distribution of infection, showing that the virus receptor is important for cell tropism as well as for adsorption to erythrocytes. During the final stages, a confluent haemorrhagic focus in the kidney is characteristic, but more frequent in North American stock. The liver lesions in affected salmon from Norway and Scotland show dilation of the hepatic sinusoids and zonal haemorrhagic necrosis with a bridge-like pattern that leaves the hepatic tissue surrounding small and medium-sized veins viable (Fig. 5.10 and 5.11). There is pronounced congestion, often combined with extensive haemorrhage in the lamina propria of the foregut and congestion of the splenic parenchyma with occasional erythrophagocytosis (see Fig. 4.11). Similarly, there can be extensive haemorrhage in the corpuscles of Stannius (Fig. 5.12). Haematological changes include vacuolation of erythrocytic cytoplasm, leucopenia and decline in haematocrit to less than 5 in severely affected fish. A differential diagnosis would be viral haemorrhagic septicaemia.
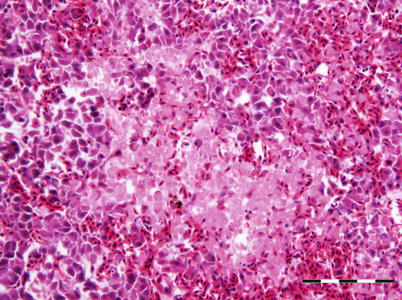
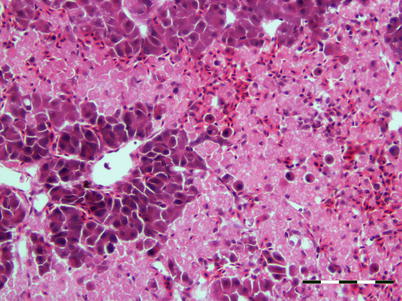
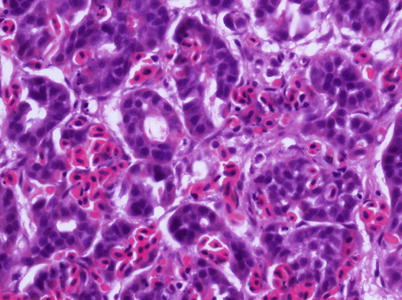

Fig. 5.10
Localised haemorrhage in head kidney from Atlantic salmon with infectious salmon anaemia. Low power

Fig. 5.11
Characteristic anastomosing liver necrosis and haemorrhage in farmed Atlantic salmon with infectious salmon anaemia. Bar scale = 100 μm

Fig. 5.12
Corpuscle of Stannius with diffuse haemorrhage in Atlantic salmon infected with infectious salmon anaemia virus. Medium power
ISA virus is a member of the Orthomyxoviridae family. Although the natural reservoir of the virus remains unknown, a marine source is considered likely. Subclinical infection has been recorded in several species including trout, rainbow trout, Arctic char, chum, Chinook and coho salmon plus marine species such as herring and pollock. Experimental data indicate that transmission may occur effectively through blood, skin mucous and faeces, with the gill capillary network being the most important route. Spread of the virus also occurs via movement of latent carriers or diseased fish, and historically through water and blood discharged from slaughtering facilities. Sea lice (Lepeophtheirus spp. and Caligus spp.) may act as vectors of the virus and the stress associated to lice infestation may make the fish more susceptible to infection. Vertical transmission of the virus has been suggested, but this is still controversial and not fully resolved.
Diagnosis is based upon characteristic clinical changes, histopathology and isolation of the virus in cell lines such as salmon head kidney (SHK-1), Atlantic salmon kidney (ASK) and CHSE. Immunohistochemistry and PCR tools are also available to demonstrate the presence of the virus, particularly in haematopoietic tissue, endocardial and chloride cells (Fig. 5.13). Variants of ISAV have been genetically differentiated on the basis of the sequence of a highly polymorphic region (HPR of genomic segment 6 which encodes the Haemagglutinin-Esterase (HE) protein). A deletion within the HPR region (named HPRΔ ISAV) in certain ISAV variants appears to be a dependable indicator of pathogenicity. ISAV without any deletions in the HPR region (HPR0 ISAV) has been reported only in apparently healthy fish and to date have not been associated with ISA disease. A reverse transcriptase-polymerase chain reaction has been developed as a sensitive method to detect carrier fish. A commercial vaccine is also available.
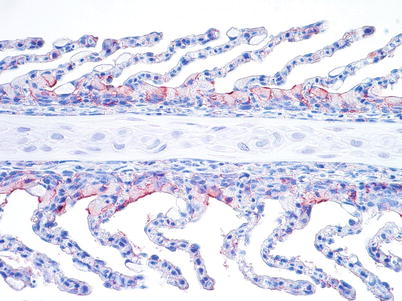

Fig. 5.13
Gill chloride cells show a strong positive reaction in farmed salmon with infectious salmon anaemia. Medium power
5.3 Oncorhynchus masou Virus
Oncorhynchus masou virus (OMV) is a virulent and economical significant disease that was originally isolated from ovarian fluids of a landlocked population of adult masou salmon in Hokkaido, Japan, but now also occurring in wild stocks. Other salmonid species are susceptible to OMV including coho, chum, kokanee and rainbow trout with high mortality in young fish.
Affected fish are dark, frequently showing severe exophthalmia and petechial haemorrhage under the lower jaw and along the ventral surface. Epithelioma around the mouth (upper and lower jaw), and, to a lesser extent, on the caudal fin, operculum and body surface occur progressively (Fig. 5.14). A white mottled appearance of the liver, progressing to a pearly white colour of the whole organ is recorded. A pale kidney and a multifocal, severe necrosis of the liver are also common. Gill epithelial cells become swollen and slough. There is a marked splenomegaly with associated necrosis of the ellipsoids and the digestive tract is generally devoid of food.


Fig. 5.14
Papillomatous neoplasia in the mandible in coho salmon due to Oncorhynchus masou virus. Bar = 100 μm
Studies involving experimental infection with OMV have shown that there is some variation in histopathological findings between species of juvenile salmon. In chum salmon, the apparent target organ is the kidney with necrosis of haematopoietic tissue, hyaline droplet degeneration and pyknotic nuclei. Partial necrosis occurs in the spleen, liver, pancreas and stomach. However, in masou salmon haematopoietic necrosis has been reported without the glomeruli or tubules being affected. OMV has oncogenic potential and induces a mandibular epithelial neoplasm in surviving fish and other neoplasms of the fins, body surface and cornea. These growths are characterised as papillomatous (Fig. 5.15). Multiple mitotic figures confirm the proliferative nature of the swelling.
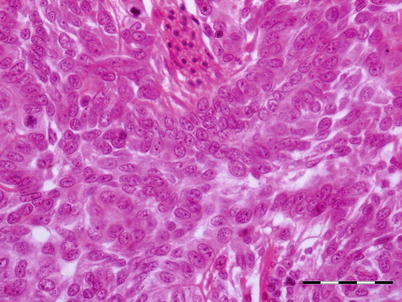

Fig. 5.15
Transverse section of papillomatous neoplasia showing proliferating epithelial cells supported by thin connective tissue in chum salmon with Oncorhynchus masou virus. Bar = 50 μm
OMV is a salmonid herpesvirus type 2 (SalHV-2) and transmitted by diseased fish and asymptomatic carriers. This virus is shed in the faeces, urine, sexual products at spawning, and probably with skin mucus. Transmission is by direct contact or through the water, but ‘egg-surface associated’ transmission probably also occurs. Symptomatic and asymptomatic carriers can spread the virus to uninfected stocks.
Diagnosis involves virus isolation using cell lines such as CHSE-214 or RTG-2 and a serum neutralisation test using a specific OMV antiserum. Viral antigens can be identified directly in tissues by immunofluorescence or ELISA. The differential diagnosis includes infectious haematopoietic necrosis, whirling disease and viral haemorrhagic septicaemia.
5.4 Piscine Reovirus (Heart and Skeletal Muscle Inflammation, HSMI)
Piscine reovirus (PRV) has been recently reported as the aetiological agent of HSMI, a systemic viral disease of sea-water farmed Atlantic salmon. The first cases were identified in Norway in 1999 and the disease is currently widespread in Norwegian aquaculture where it causes substantial losses. This condition has also been described from farmed salmon in Scotland. The PCR-screening of marine fish caught along the Norwegian coast has revealed PRV in great silver smelt, capelin, Atlantic herring and horse mackerel.
Clinical outbreaks typically occur 5–9 months after transfer to sea water. Morbidity may be very high in affected cages, while mortality may reach 20 %. Clinical signs include anorexia and abnormal swimming behaviour and internally, pale heart, yellow-orange liver, ascites, splenomegaly and visceral petechiae.
Characteristic histopathological changes are found in heart and skeletal red muscle. Red skeletal muscle is usually heavily affected with myocyte degeneration and infiltration of inflammatory cells (Figs. 5.16 and 5.17). In the heart, early lesions in the ventricular compactum typically include perivasculitis associated with branches of the coronary vessels, endocarditis and focal myocarditis (Fig. 5.18). A highly cellular epicarditis can also be observed (Fig. 5.19). Cardiac lesions subsequently spread to the entire myocardium developing an extensive panmyocarditis, multifocal necrosis and inflammation dominated by neutrophils and macrophages in both spongy and compact myocardium, within and between muscle fibres, and aggregates or ‘nests’ of small nuclei may be seen in affected myocardium. Additional cardiac lesions are compensatory karyomegaly and show elongated Anitschkow-like nuclei. Atrial lesions are similar to those seen in the spongy myocardium, but often milder.
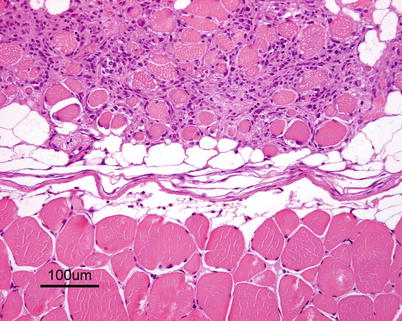
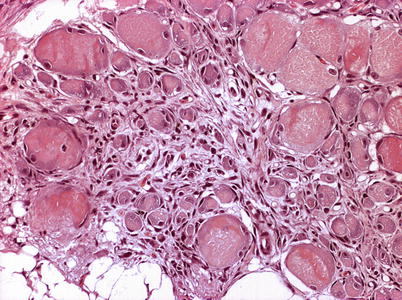
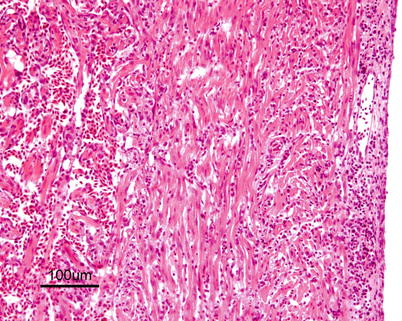
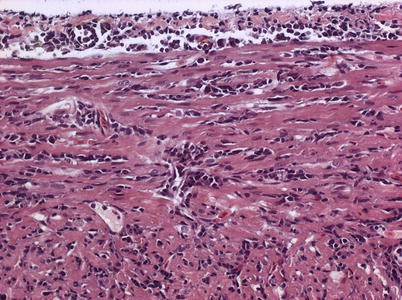

Fig. 5.16
Degeneration and inflammation of red muscle (top) in Atlantic salmon with heart and skeletal muscle inflammation

Fig. 5.17
Transverse section showing degeneration and inflammation of red muscle in farmed Atlantic salmon with heart and skeletal muscle inflammation. Medium power

Fig. 5.18
Heart and skeletal muscle inflammation in farmed Atlantic salmon. Severe inflammation in both myocardial layers and epicardium

Fig. 5.19
Cellular epicarditis and inflammation of compact myocardium in farmed Atlantic salmon with heart and skeletal muscle inflammation. Medium power
Lesions in other organs are few but general congestion and multifocal liver necrosis with vacuolated and pyknotic or karyolytic cells may be seen. In addition, haemorrhage and accumulation of erythrocytes can be recorded in gills, kidney and spleen.
PRV belongs to the reovirus group and appears to be widespread in farmed salmon. The route of infection is presently unknown. However, a low prevalence in several non-salmonid species from Norwegian waters using a real-time PCR suggests there is a complex relationship that involves carriers and virus reservoirs. Diagnosis comes from the characteristic histopathological lesions in heart and red muscle, immunohistochemistry and the presence of PRV demonstrated by PCR. Pancreas disease (PD) and cardiomyopathy syndrome (CMS) are important differential diagnoses, but may be differentiated from HSMI by the type and distribution of cardiac lesions, pancreatic lesions and pathological changes in red muscle. There is no treatment or vaccines available. Prophylactic measures include reduction of stress and restrictions on the transport of live fish.
5.5 Salmon Leukaemia Virus
Plasmacytoid leukaemia (PL) of farmed Chinook on the west coast of North America is attributed to salmon leukaemia virus (SLC). Evidence of PL has also been recorded in freshwater-reared salmon in the United States and in salmon from net-pens in Chile. Other salmonids such as coho, sockeye and Atlantic salmon are considered susceptible under experimental conditions. Affected fish are dark, lethargic and often show severe bilateral exophthalmia. Pale gills are a regular finding and affected fish usually stay near the water surface. Spleen, kidney and retrobulbar tissue are enlarged and mottled, with petechial haemorrhage in several organs including the liver, mesenteric fat, pancreas and skeletal muscle. Histologically, infection is characterised by proliferation and infiltration of plasmablasts and other lymphoid cells into the visceral organs and retrobulbar tissues (Figs. 5.20 and 5.21). The plasmablasts have lobate nuclei and prominent nucleoli. The kidney shows mild to moderate hyperplasia of the haematopoietic interstitium. Evidence suggests this is a neoplastic condition rather than a reactive plasmacytosis. There is some indication that the presence of an intranuclear microsporidian, Nucleospora salmonis (previously Enterocytozoon salmonis) is associated with PL, and maybe a cofactor. The diagnosis of plasmacytoid leukaemia (a retrovirus) is provisionally based upon the detection of a large number of plasmablasts and supported by an IFAT using tissue imprints.