, Patricia A. Noguera1 and Trygve T. Poppe2
(1)
Marine Scotland Science, Aberdeen, UK
(2)
Norwegian School of Veterinary Science, Oslo, Norway
Abstract
Bacterial infections comprise true obligate and opportunistic facultative pathogens. The distinction between ‘primary’, ‘facultative’ or ‘opportunistic’ cannot be taken too strictly as the virulence of the agent will determine its ability to overcome the host defences. Bacterial diseases are largely represented by Gram negative organisms and affect salmonids in fresh, sea water or both. A few important diseases of salmon and trout, both in fresh and sea water are caused by Gram positive bacteria. This chapter covers a wide range of bacterial diseases representing acute, chronic, systemic or localised infections affecting salmonids in fresh and sea water.
Keywords
BacteriaInfectionSalmonTroutBacteria exist everywhere and the majority are capable of independent existence for varying periods without a ‘host’. Specific bacteria cause disease, either because they are ‘designed’ to invade a host (true obligate pathogens) or simply because they are in the wrong place at the right time (opportunistic facultative pathogens). The distinction between ‘primary’, ‘facultative’ or ‘opportunistic’ cannot be taken too strictly, as the virulence of the agent will determine its ability to overcome the host defences. These vary between fish stock and circumstances including stress, water quality or other co-existing infections. When a bacterium is present in a host and associated with a disease condition, it will normally be deemed a ‘primary pathogen’, while those agents that can be found without compromising the fish health but are capable of inducing disease under certain conditions, will be addressed as ‘facultative pathogens’. When bacteria survive and multiply in the host tissues without causing clinical disease, fish harbouring such bacteria are known as ‘asymptomatic carriers’. The pathology and outcome of a bacterial infection may vary and dependant on factors linked to the bacterium, the host and/or the environmental conditions.
Bacteria gain entry to the fish through the gills, gut or via the skin, and then usually spread throughout the body. As infections become systemic they can induce acute changes that externally result in exophthalmia, hyperaemia and petechial haemorrhage, while internally, ascites, congestion and haemorrhage can be observed. However, some bacteria cause chronic infections with tissue proliferation and repairing processes, resulting in typical granulomatous responses.
Bacterial diseases are mainly represented by Gram negative organisms such as Aeromonas salmonicida, Listonella anguillarum and Yersinia ruckeri, affecting salmonids in fresh, sea water or both. Some important diseases however are caused by Gram positive bacteria, e.g. Renibacterium salmoninarum in salmon and trout, both in fresh and sea water. The Chlamydiales and Rickettsiales contain the genera Chlamydia and Piscirickettsia respectively, and are obligate intracellular pathogens which multiply within membrane-bound cytoplasmic vacuoles. Most of these infections occur in marine or anadromous hosts, but they have also been reported in freshwater fish. Measures to limit or control outbreaks in farmed fish include the use of vaccines and antimicrobial agents respectively.
Taxonomy has progressed dramatically in the last decade moving from culture-dependent techniques involving phenotype characterization and physiological data, to the application of molecular techniques, 16S rRNA and gene sequencing. While the traditional methods of culture remain relevant, new techniques are contributing to an accurate and rapid diagnosis of certain agents. Principal and emerging pathogens are listed in Table 6.1.
Table 6.1
Principal and emerging bacterial and chlamydial diseases of salmonids
Gram negative bacteria | Disease | `Principal salmonid host | Environment |
|---|---|---|---|
Aeromonas hydrophila | Rainbow trout | FW | |
Aeromonas salmonicida subsp. salmonicida | Furunculosis | Most salmonids | FW |
Listonella anguillarum | Vibriosis | Salmonids | SW |
Aliivibrio salmonicida, A. wodanis, A. logei | Cold-water vibriosis | Atlantic salmon | SW |
Moritella viscosa | Winter ulcer disease | Atlantic salmon | SW |
Yersinia ruckeri | Enteric redmouth | Rainbow trout | FW |
Pseudomonas fluorescens | Rainbow trout | FW | |
Flavobacterium psychrophilum | Rainbow trout fry syndrome, peduncle disease | Rainbow trout | FW |
Flavobacterium columnare, hydatis/johnsoniae | Columnaris disease | Rainbow trout | FW |
Tenacibaculum maritimum | Black patch necrosis, marine flexibacteriosis | Atlantic salmon | SW |
Hafnia alvei | Rainbow trout | FW | |
Chryseobacterium spp. | Rainbow trout | FW | |
Pasteurella skyensis | Atlantic salmon | SW | |
Francisella noatunensis supsp. noatunensis | Atlantic salmon | SW | |
Gram positive bacteria | |||
Renibacterium salmoninarum | Bacterial kidney disease | Rainbow trout/salmon | FW, SW |
Carnobacterium maltaromaticum (synonym C. piscicola) | Pseudokidney disease | Rainbow trout/chinook salmon | FW |
Streptococcus phocae | Atlantic salmon | SW | |
Acid fast bacteria | |||
Mycobacterium chelonae, M. fortuitum, M. marinum | Mycobacteriosis | Salmon | SW |
Nocardia sp. | Nocardiosis | ||
Chlamydiaceae | |||
Piscirickettsia salmonis | Salmonid rickettsial septicaemia | Salmon | SW |
Candidatus Piscichlamydia salmonis, Candidatus Clavochlamydia salmonicola | Epitheliocystis | Atlantic salmon, Arctic char, brown trout | FW, SW |
Candidatus Branchiomonas cysticola | Epitheliocystis | Salmon | SW |
Candidatus arthromitus | Rainbow trout gastroenteritis | Rainbow trout | FW |
6.1 Aeromonas hydrophila
Aeromonas hydrophila is a widely distributed fresh water bacterium and the causative agent of a condition known as ‘motile aeromonad septicaemia’. The bacteria affects wild and farmed fish species and aggravated by poor water quality principally when the water temperature is above 10 °C
A. hydrophila was significant for farmed fish during the 1970s until the emergence of successful vaccines during the 1980s. Nevertheless, it remains an important pathogen and clinical outbreaks are sporadically recorded. External signs include darkened skin, abdominal distension and exophthalmia, with erosion and apparent necrosis near the tail and other fins. Gills can be haemorrhagic or pale and swollen, with haemorrhage also seen in the vent and large areas of the skin, where oedema of superficial lesions may develop and ulcerate which then become prone to secondary infections e.g. Saprolegnia.
Internally, there is evidence of anaemia and the accumulation of clear or blood-tinged ascites. Splenomegaly and swollen kidney are common. Histopathological changes are characteristic of a generalised septicaemia with focal lesions involving several tissues such as gill, brain, heart, intestine, kidney and liver. A liquefactive necrosis and haemorrhage is detected in liver and kidney, with serous exudates in the intestine. The disease shows similar characteristics to those seen in a pseudomonad infection (see Pseudomonas fluorescens ). Diseased fish transmit the infection horizontally through the water.
Aeromonas hydrophila is a motile, fermentative Gram negative rod which produces catalase and cytochrome oxidase. Material taken from the kidney and other organs onto a non-selective media is generally sufficient to obtain growth. Diagnosis is based upon clinical disease signs, and the isolation and identification of the bacteria on medium such as trypticase soy agar (TSA) using morphological characteristics.
6.2 Aeromonas salmonicida subsp. salmonicida
Furunculosis is the name used to describe infections with Aeromonas salmonicida subsp. salmonicida. Infection occurs in wild and farmed salmonids, and has been recorded in Atlantic salmon, brown trout, Arctic char, brook trout and lake trout. Both chronic and acute furunculosis may occur depending on water temperature, fish age and virulence of the agent. Outbreaks are often triggered by stressors such as changes in temperature, poor handling, water quality and crowding.
In acute infections fish may die with few or no signs of disease or pathological changes. During the chronic stages the fish show lethargy, inappetence and darkening of the skin, which is similar to most bacterial septicaemias. Ventral haemorrhage is common, in particular near the base of the pectoral, pelvic and anal fins in addition to exophthalmia. At necropsy, ascites, splenomegaly, haemorrhagic enteritis, ascites, sub-capsular liver haemorrhage and pyloric caeca may be observed. Liquefactive, haemorrhagic ‘boil’ lesions that develop in the flank skeletal muscle are observed in chronic cases (Figs. 6.1, 6.2, 6.3 and 6.4). These ‘furuncles’ may rupture exposing open deep ulcers on the surface A large number of bacteria are released from these lesions and contribute to the spread of the infection. Although furuncles are characteristic, they are not always present in diseased fish and cannot be regarded as a diagnostic feature. A carrier state may be established after an infection.


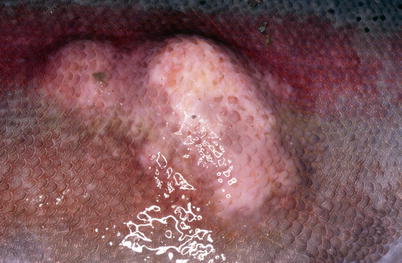
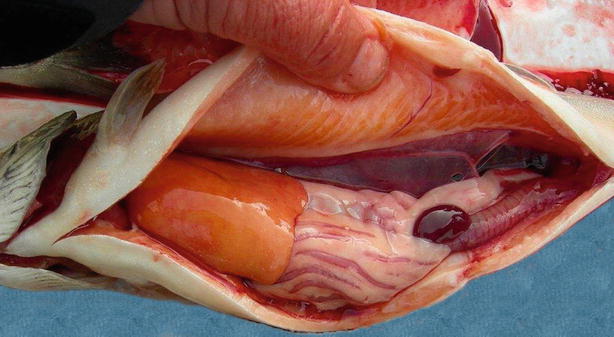

Fig. 6.1
Furunculosis in farmed brook trout; mild ulcerated subcutaneous boil lesion

Fig. 6.2
Furunculosis in adult sockeye salmon; subcutaneous boil lesion in ventral muscle

Fig. 6.3
Furunculosis in farmed rainbow trout broodstock; subcutaneous boil lesions

Fig. 6.4
Furunculosis in farmed Arctic char; ascites and swollen spleen
Histopathological lesions are characterized by dense aggregates of bacteria in organs such as heart, kidney, spleen, muscle and gills (Figs. 6.5 and 6.6). In the latter, embolic spread with subsequent bacterial colonization may be seen in the gill capillaries. Mural thrombi with bacterial colonization are observed on the intimal aspect of vessels and ellipsoids. There is remarkably little tissue reaction around aggregates of bacteria in early stages of the disease, but tissue necrosis and liquefaction may become extensive in late and chronic stages.
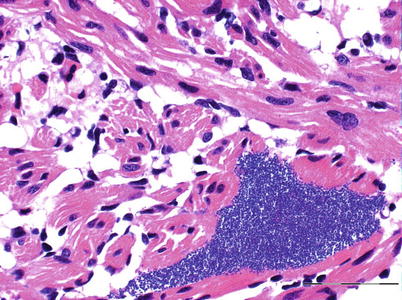
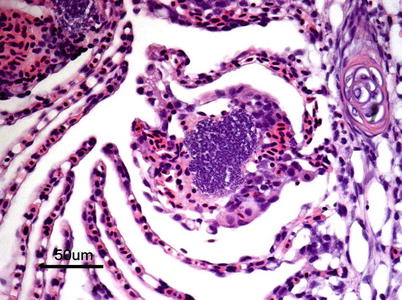

Fig. 6.5
Furunculosis in rainbow trout; microcolony in spongy myocardium. Medium power

Fig. 6.6
Furunculosis in farmed Atlantic salmon, microcolony in lamella
Aeromonas salmonicida subspecies salmonicida is a Gram negative, non-motile, facultative anaerobic rod. Pathogenicity is dependent on the so-called A-layer, an external surface layer mainly composed of the A-protein that provides protection against the defence mechanisms of the host. At least 25 extracellular products are released during growth and responsible for the tissue damage-degeneration and ultimately, the death of the fish. Primary isolation of the pathogen can be achieved from the kidney and other organs on TSA or brain heart infusion agar (BHIA) at 22 °C. Most strains are oxidase positive and produce a water-soluble brown pigment on medium containing tryptone. A less common and atypical strain of this pathogen is the subspecies achromogenes, which does not produce pigment under standard incubation conditions and requires additional biochemical testing to differentiate. Diagnosis of A. salmonicida is based upon gross and histopathological lesions, isolation of the causative agent or its identification through immunohistochemistry, serology or molecular tools. In haematoxylin and eosin (H&E) sections the appearance of the bacteria is almost pathognomonic (see Fig. 6.5).
6.3 Listonella anguillarum
Listonella (Vibrio) anguillarum causes a haemorrhagic septicaemia, an economical important disease affecting salmonids in salt and brackish waters, particularly in the summer months at temperatures above 10 °C. However, infections with Vibrio ordalii and other Vibrio spp. are often collectively termed ‘vibriosis’, and may cause disease with similar clinical and pathological manifestation as L. anguillarum. Clinical signs and pathological lesions may be variable, but similar to several other Gram negative septicaemias, and dependant on water temperature, fish age and pathogen virulence. L. anguillarum may be present among the normal gut microflora of healthy fish and clinical outbreaks triggered only by stress activated virulent strains present in the gastrointestinal tract. Poor water quality and rapid temperature changes can also activate infection which then spreads horizontally. External signs of disease include dark skin coloration, anorexia, pale gills with increased mucous, periorbital oedema, swollen vent (Fig. 6.7) and haemorrhage near the base of the pectoral and pelvic fins. V. ordalli shows a predilection for muscle and skin with resulting haemorrhage. Extensive multifocal liquefactive muscle necrosis and haemorrhage with large numbers of bacteria is common (Fig. 6.8). Dermal or subdermal skin lesions are often coupled to hyperaemia and haemorrhage, and may be linked to occasional haemorrhagic ‘boil’ lesions in the muscle. These may rupture and release blood and bacteria to the surrounding water. Internally, petechiae may be present on the peritoneal surface and in internal organs. The liver is generally swollen and some fish show petechiae (Fig. 6.9). Colonies of the bacterium are also found throughout the digestive tract and in loose connective tissues such as in the gills. Bacteria can also be found at the back of the eye (Fig. 6.10), with opacity followed by corneal lesions, ulceration and avulsion of the orbital contents. Splenomegaly is common and even rupture of the organ may occur, particularly in rainbow trout. Histologically, the anterior part of the digestive tract may show vasodilatation and extensive necrosis of the mucosa and muscularis. Necrosis and oedema of haematopoietic tissue and spleen can also be found. A haemolytic anaemia occurs in chronic cases with resulting deposition of haemosiderin in the melanomacrophage centres of the splenic ellipsoids.
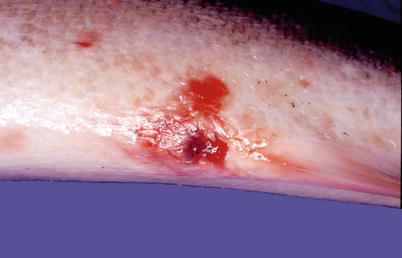
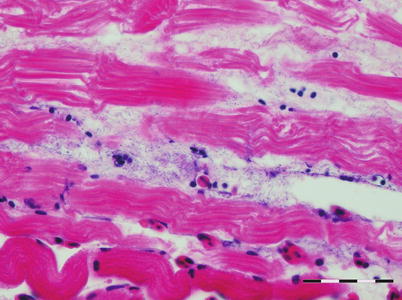
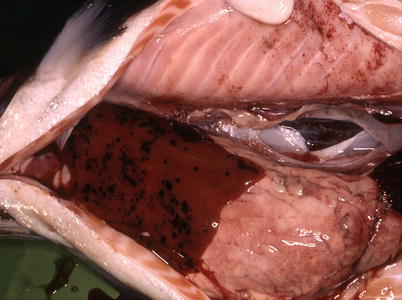
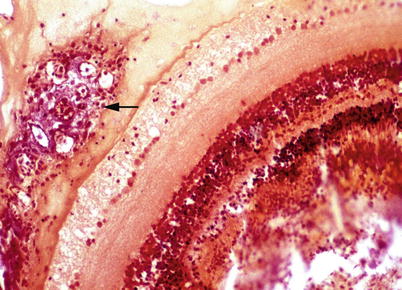

Fig. 6.7
Vent haemorrhage in Atlantic salmon with Vibrio sp. infection

Fig. 6.8
Listonella anguillarum infection in muscle lesion in Atlantic salmon. Bar = 50 μm

Fig. 6.9
Petecchia in liver and peritoneum of farmed Atlantic salmon resulting from Listonella anguillarum infection

Fig. 6.10
Listonella anguillarum infection in rainbow trout. Retrobulbar proliferation of bacteria (arrow). Gram stain. Low power
Listonella anguillarum is a halophilic with bipolar staining, Gram negative, slightly curved, flagellated motile rod. The bacterium grows well on standard medium at an optimum temperature of 22 °C and shows haemolytic activity on blood agar. Serovars O1 and O2 are the most common causing infection in salmonids, but more than 20 different serovars have been described.
Diagnosis is based upon classical pathology and isolation of L. anguillarum on NaCl-supplemented blood agar or TSA at room temperature and confirmation by ELISA. Serologically identification can be carried using a rapid agglutination test kit. Oil-adjuvant multivalent vaccines usually provide excellent protection against both O1 and O2 serovars of L. anguillarum.
6.4 Aliivibrio (Vibrio) salmonicida
Cold water vibriosis (Hitra disease or haemorrhagic syndrome) is a septicaemic condition of farmed Atlantic salmon caused by the motile bacterium Aliivibrio (Vibrio) salmonicida. The first observations were recorded in northern Norway in 1977 and the disease was a serious threat to the rapidly growing farming industry in the early 1980s. Outbreaks have also been recorded in Scotland, Faroe Islands, Iceland and the east coast of USA and Canada. Currently, oil-adjuvant multivalent vaccines give excellent protection against cold-water vibriosis and overall the impact of the disease has been greatly reduced.
Cold-water vibriosis typically occurs during the winter months. Affected fish go off the feed, are lethargic and dark coloured and often stay near the surface. As with some other diseases of farmed salmonids, apparently ‘healthy fish’ are often heavily affected. External lesions include exophthalmia, a swollen and haemorrhagic vent, petechial haemorrhage under the belly and at the base of the pectoral and pelvic fins. Necropsy may reveal a yellowish liver, sometimes with petechial or ecchymotic haemorrhage (Fig. 6.11) ascites, splenomegaly, haemorrhagic enteritis and a generalised oedema. Petechiae in the pyloric region are also common. Early histopathological lesions are characterized by large numbers of bacteria in blood vessels, followed by the heart, kidney, muscle and spleen, but with little tissue reaction (Fig. 6.12). Congestion with arteriole mural necrosis and thrombi are recorded, as well as kidney tubular necrosis and myolysis of skeletal muscle in the latter stages. Viral septicaemia may be an important differential diagnosis.
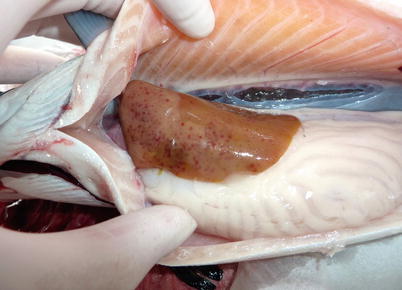
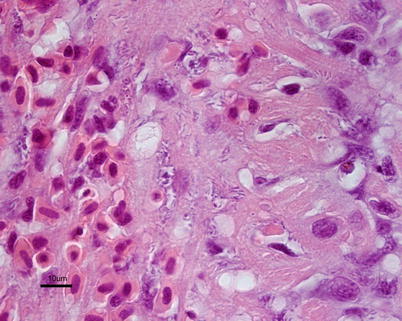

Fig. 6.11
Aliivibrio wodanis infection in farmed Atlantic salmon. Anaemic fish with pale liver and petecchia

Fig. 6.12
Diffuse infiltration with Aliivibrio salmonicida in spongy myocardium of farmed Atlantic salmon with cold-water vibriosis. High power
Diagnosis is based upon gross lesions, histopathology and the isolation of Aliivibrio (Vibrio) salmonicida. The bacterium is a psychrophilic, moderately halophilic Gram negative curved or straight rod, and is motile with up to 9 polar sheathed flagella. It can be grown at 15 °C on NaCl-supplemented blood agar where optimum salt concentration is 1.5 %. Growth shows small, greyish, non-haemolytic colonies on blood agar that are facultative anaerobic, oxidase positive and susceptible to the vibriostatic agent 0/129.
6.5 Moritella viscosa
Moritella viscosa is one of the causative agents of ‘winter ulcers’, a commonly occurring skin disease of farmed salmon and rainbow trout that has been diagnosed in Norway, Iceland, Faroe Islands and Scotland. M. marina has also been described. Infection results in increased mortality rates and major economic losses due to downgrade of the fish at slaughter, and typically occurs at a low but consistent prevalence in farmed Atlantic salmon during the coldest months of the year. The significance of the disease is associated to poor animal welfare, reduced osmoregulatory capacity, increased susceptibility to other infections and reduced marketability. Clinical observations show small raised skin lesions on the flank of the fish. These increase in size and gradually break the skin exposing the underlying muscle (Fig. 6.13). Lesions are characteristically rounded or oval with a white demarcation zone towards normal skin, which may heal with increasing temperature leaving ‘scar’ tissue, sometimes with melanisation of the area. The infection may become systemic and extensive petechial haemorrhage may develop in the ventral surface (Fig. 6.14) and internally in the peritoneum, adipose tissue, pyloric region and liver (Fig. 6.15).

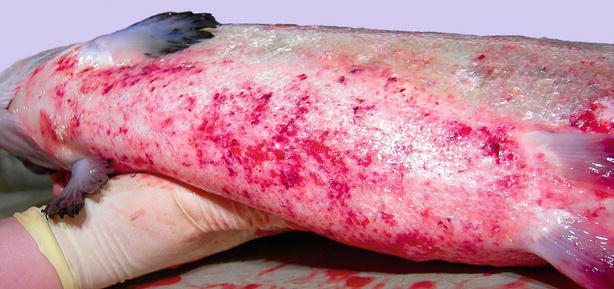
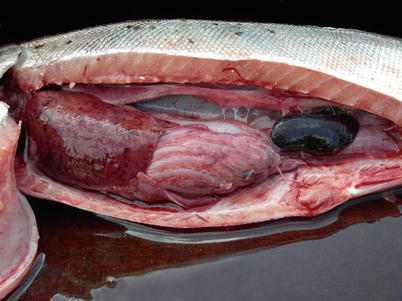

Fig. 6.13
Winter ulcers caused by Moritella viscosa in farmed Atlantic salmon

Fig. 6.14
Ventral haemorrhage in farmed Atlantic salmon with systemic Moritella viscosa infection

Fig. 6.15
Liver haemorrhage and swollen spleen in farmed Atlantic salmon with systemic Moritella viscosa infection
Histopathological changes are variable depending on the period of ulcer development. Initial stages are characterized by oedema down to the compact layer of dermis and some inflammatory cell invasion. Ata later stage, lesions may reach the white muscle with inflammatory infiltrates between muscle bundles, haemorrhage and thrombosis of small vessels. Bacteria are typically found near the edges of the lesions. In the reparative phase, granulation tissue covers the ulcers starting from the edges and is gradually replaced by new epidermal and dermal layers, without scales. Diagnosis is based upon clinical signs, and the isolation and identification of the bacteria. A differential diagnosis would include other Vibrio spp.
Moritella viscosa is a psychrophilic Gram negative, motile, flagellated curved rod. M. viscosa antigens are included in most of the multivalent injection vaccines used in the salmon industry, but the protection is apparently variable.
6.6 Yersinia ruckeri
Yersinia ruckeri is the causative agent of yersiniosis or enteric redmouth disease (ERM), an economically important condition in wild and farmed salmonids, both in fresh and sea water. Y. ruckeri has a wide host range and most salmonids are susceptible. Transmission occurs horizontally and many species of asymptomatic carriers as well as birds, being reservoirs of infection. Outbreaks are typically stress-mediated, e.g. poor water quality, increase in temperature, grading and handling. Clinical signs and pathology are similar to other Gram negative septicaemias and the disease may occur in peracute to acute or more chronic forms. In peracute cases, as in fry or fingerlings in freshwater, there may be high mortality with few or no external disease signs. In the more chronic cases, fish show pigment changes, disturbance of balance and lethargy. Other signs include ascites, exophthalmia, cutaneous petechiae and localized haemorrhage at the tip of the gill filaments. Hyperaemia of the oral cavity and jaw as a result of congestion of the submucosa, is not always evident, but has given the disease its popular name, enteric redmouth disease (Fig. 6.16). At necropsy, general congestion, intestinal haemorrhage, petechiae on serosa membranes, a swollen kidney and splenomegaly are common (Fig. 6.17). Histologically, haemorrhage, congestion, oedema and bacterial colonization of several tissues, including brain and gills is frequent. Necrosis associated with bacterial colonization is common in the kidney particularly the glomerulus, and the spleen (Fig. 6.18).

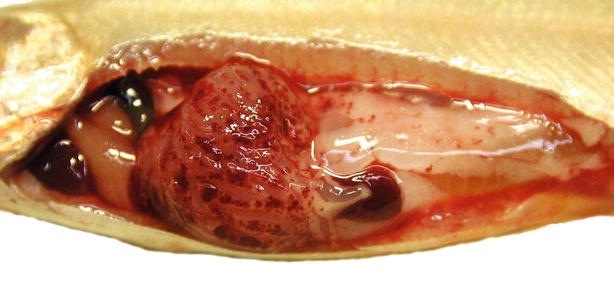
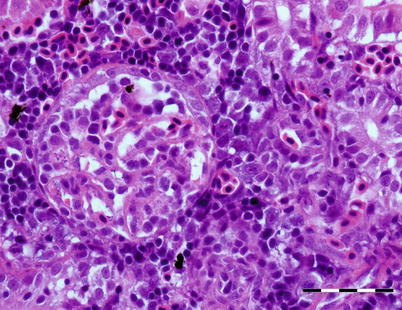

Fig. 6.16
Mandibular haemorrhage in rainbow trout fingerlings infected with Yersinia ruckeri

Fig. 6.17
Enteric redmouth in farmed rainbow trout; petecchia in peripancreatic tissues

Fig. 6.18
Bacteria in glomerular vessels and thickened basal membrane in rainbow trout infected with Yersinia ruckeri. Bar = 50 μm
As gross and histopathological changes may be unspecific, the diagnosis requires confirmation by isolation of the causative agent on TSA or blood agar at 22 °C. Y. ruckeri is a Gram negative, motile rod-shaped bacterium. It is catalase positive and oxidase negative and several serotypes have been identified. On TSA Y. ruckeri colonies are rounded, translucent, glistening and buff-coloured colonies after 24 h incubation. Immunohistochemistry, fluorescent antibody and ELISA can be used for confirmation (Fig. 6.19). Both immersion and injection vaccines are available and provide good protection against clinical outbreaks.
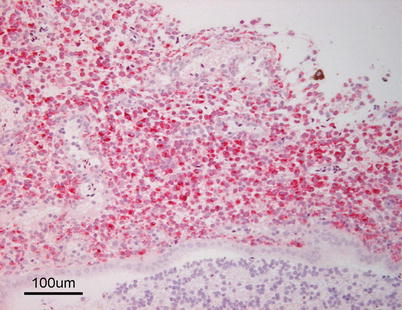

Fig. 6.19
Meningitis in farmed rainbow trout with enteric redmouth. Immunohistochemical stain
6.7 Pseudomonas fluorescens
The ubiquitous bacterium Pseudomonas fluorescens is generally considered as a non-pathogenic saprophyte, however, it can become an opportunistic pathogen causing disease in wild and farmed salmonids, particularly following stress and periods of poor water quality. Under farming conditions outbreaks can occur after vaccination or concurrent to other disease (e.g. infectious pancreatic necrosis). Smolts suffering from subclinical disease during smoltification and sea-water transfer may show clinical disease during the sea water phase. Affected fish may display many different manifestations of disease, from chronic, non-symptomatic, to acute haemorrhagic septicaemia, with high mortality (>15 %). External signs may include exophthalmia, frayed fins or fin rot, skin ulcerations with haemorrhagic edges, ventral petechiae and dark colouration (Fig. 6.20). At necropsy, changes are similar to other bacterial conditions, including ascites, petechiae on internal organs and pale liver, but a purulent epicarditis can also be observed (Fig. 6.21).




