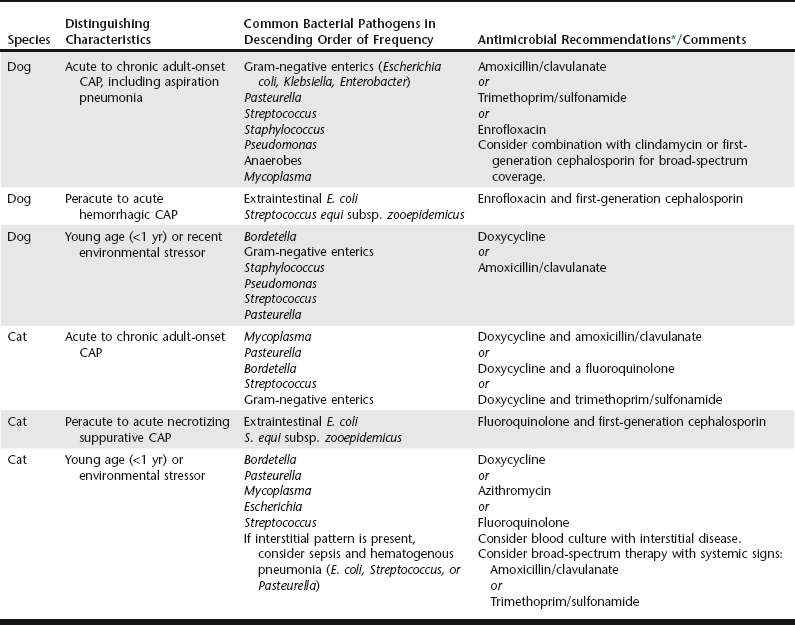
Chapter 162 Community-acquired pneumonia (CAP) is defined as pneumonia that develops in a patient that has not recently been hospitalized. The incidence of CAP in dogs and cats is unknown. Infection of the lung may occur by aspiration, inhalation, or hematogenous spread of virulent bacteria or opportunistic pathogens in immunosuppressed individuals. The different forms of CAP with distinguishing characteristics and pathogens are summarized in Table 162-1. TABLE 162-1 Community-Acquired Pneumonia (CAP) and Early-Onset Hospital-Acquired Pneumonia: Distinguishing Characteristics, Common Bacterial Isolates, and Antimicrobial Recommendations *Avoid fluoroquinolones in puppies. Use caution with trimethoprim/sulfonamide and monitor patients for ocular or hematologic abnormalities. Aspiration (discussed in more detail later) and opportunistic organisms appear to be more common causes of infection than virulent bacteria in adult dogs and cats. Causes of immunosuppression that are associated with pneumonia in mature animals include preexistent disease (e.g., diabetes mellitus, hypothyroidism, neoplasia, or bronchiectasis) and therapeutic immunosuppression related to treatment of primary or acquired immunologic conditions (e.g., immune-mediated hemolytic anemia, lupus, or pemphigus). In a report of a case series describing bacterial pneumonia in 93 dogs, most affected animals were older than 5 years of age and 57% had concurrent medical problems that may have contributed to aspiration or immunosuppression (Jameson et al, 1995). Bacterial cultures of specimens from the level of the carina yield oropharyngeal bacteria in up to 40% of healthy dogs and 75% of healthy cats (Box 162-1), and some of these organisms have been associated with pneumonia in dogs with aspiration injury or other causes of compromised immune function. Infection in young animals without concurrent illness is often associated with exposure to more virulent organisms, but a significant stressor (e.g., housing, transport, or environmental) may contribute to some degree of immune dysfunction and compromised resistance to infection. Some pathogens causing CAP establish colonization with specialized virulence factors, regardless of the immune status of the patient. Some examples are extraintestinal pathogenic Escherichia coli and Streptococcus equi subsp. zooepidemicus, which have been responsible for hemorrhagic or necrotizing pneumonia in dogs and cats. Outbreaks of S. equi subsp. zooepidemicus also have been reported to cause fatal hemorrhagic pneumonia in dogs (Byun et al, 2009; Priestnall and Erles, 2010) and necrotizing suppurative pneumonia in cats (Blum et al, 2010). These organisms typically result in peracute to acute infections, the animals are profoundly ill with pyrexia and tachypnea, and mortality is high. Most affected animals had experienced a stressor, such as shipment or housing in a facility or shelter, that may have contributed to increased host susceptibility. Another cause of CAP in both dogs and cats is infection with Bordetella bronchiseptica. This gram-negative aerobic coccobacillus has the capacity to colonize the airway as either a commensal or a pathogenic organism, and it frequently plays a role in canine infectious respiratory disease complex (see Chapter 153). The outcome of infection is difficult to predict and can range from mild subclinical disease to fatal pneumonia. B. bronchiseptica displays a myriad of virulence factors that influence the pathologic consequences of infection and may be affected by various environmental conditions. In a case series of puppies with CAP, B. bronchiseptica was isolated in 49% (32 out of 65) and predominantly gram-negative enteric bacteria were isolated in the rest, all of which were susceptible to most antimicrobials tested (Radhakrishnan et al, 2007). Mycoplasma spp. are considered a normal inhabitant of the oropharynx of both dogs and cats, and they have been cultured from the lungs of many animals with pneumonia; however, most cases showed resolution of clinical disease without specific anti-Mycoplasma therapy (Jameson et al, 1995). This has led to speculation that these organisms may be an incidental contaminant or that they may have low pathogenicity on their own. However, Mycoplasma spp. also have been recovered as the sole pathogen from the airways of animals that subsequently responded to anti-Mycoplasma therapy. Several of those animals had findings consistent with bronchitis, asthma, or airway collapse, and whether Mycoplasma contributed to airway disease or was able to colonize as a downstream consequence of this disease is unknown. At present it seems likely that colonization of the lower respiratory tract by the organism does have clinical significance that warrants therapeutic consideration, especially in the cat. Hospital-acquired pneumonia (HAP) is defined as pneumonia that occurs 48 hours or longer after admission and was not incubating at the time of admission (American Thoracic Society and Infectious Diseases Society of America, 2005). HAP can be used synonymously with nosocomial pneumonia and is the second most common nosocomial infection in humans in the United States. HAP in humans accounts for up to 25% of all intensive care unit (ICU) infections and more than 50% of antibiotics prescribed, and is associated with morbidity and mortality rates of 33% to 50%. Although the incidence in dogs and cats is unknown, in the authors’ opinion HAP is a significant problem in veterinary referral hospitals and needs to be considered separately from CAP. In humans the length of time from admission to development of HAP has implications for therapy. Early-onset HAP, defined as HAP occurring within the first 4 days of hospitalization, typically carries a better prognosis because it is more likely to be caused by antibiotic-sensitive bacteria. Late-onset HAP, defined as HAP occurring after 5 days of hospitalization or longer, is more likely to be caused by multidrug-resistant (MDR) pathogens and is associated with increased morbidity and mortality. In veterinary medicine, there are few studies that specifically address HAP. Nevertheless, veterinarians are finding similar patterns of MDR bacterial infections in our patients. One report described antimicrobial therapy and susceptibility patterns in isolates from various sources (urinary, respiratory, peritoneal) obtained from animals in a veterinary ICU (Black et al, 2009). MDR pathogens comprised 27% of isolates (19 out of 70) and were significantly more likely to be identified after 48 hours of hospitalization. Initial empiric antibiotic selection was appropriate in only 30% of animals that had received antibiotics in the month before culture submission. Antibiotics prescribed after submission of the sample but before final bacteriologic culture and susceptibility (minimal inhibitory concentration) results became available were appropriate only 75% of the time (Black et al, 2009). These findings underscore the importance of identifying antimicrobial susceptibility patterns in patients in the ICU. A 2010 case series (Epstein et al, 2010) compared airway microbial culture and susceptibility patterns in dogs and cats in the ICU that required positive pressure ventilation due to respiratory failure with findings in animals with respiratory infection that did not require intensive care during the same time period. Although the authors of that report did not specifically differentiate CAP from HAP, there was a clear distinction in severity of disease and level of care. In animals with respiratory failure gram-negative enteric organisms were more likely to be isolated, whereas in animals with respiratory infection gram-negative nonenteric and anaerobic organisms predominated. Fewer bacterial isolates from the respiratory failure group than from the respiratory infection group were susceptible to amoxicillin/clavulanate (35% versus 84%, respectively), enrofloxacin (33% versus 69%, respectively), and ticarcillin/clavulanate (47% versus. 83%, respectively). The findings described in these reports suggest that the historical recommendation to prescribe therapy based on bacterial identification alone is likely inadequate in the setting of HAP. Animals with late-onset HAP or other risk factors for MDR pathogens (Box 162-2) should be treated with antibiotics that are more likely to be effective against MDR organisms (Table 162-2) while awaiting susceptibility results. TABLE 162-2 Antimicrobial Recommendations for Patients with Late-Onset Hospital-Acquired Pneumonia or Patients at Risk of Infection with Multidrug-Resistant Pathogens Aspiration pneumonitis is a chemical injury caused by the inhalation of sterile gastric contents, whereas aspiration pneumonia is an infection caused by the inhalation of oropharyngeal or gastric contents that are contaminated by pathogenic bacteria. The incidences of both are unknown in veterinary medicine. The true incidence in humans also has been difficult to establish because there are no sensitive and specific markers for aspiration. If the patient does not have a reason for gastric colonization (Box 162-3) and has good oral hygiene, the initial aspiration event should be nearly sterile and acidic in nature, leading to a biphasic lung injury. The first phase, occurring in 1 to 2 hours, is secondary to direct injury of pulmonary parenchyma from acidic stomach contents. The second phase is typical of acute lung injury and develops as neutrophils infiltrate the alveoli as part of the postinjury inflammatory process. This host defense response, which is essential to remove inhaled particulate matter, begins several hours after the event and can progress over the next 24 to 48 hours. This phase represents true pneumonitis that does not require initial antimicrobial therapy. In fact, administration of antibiotics may tend to select for more resistant organisms if a secondary infection is established. However, many animals do not have good oral hygiene, and many of the disease processes leading to the aspiration event are treated with therapies that contribute to gastric colonization, including proton pump inhibitors and enteral nutrition (see Box 162-3). Therefore treatment of aspiration injury in dogs and cats should follow the same recommendations as those for treatment of uncomplicated CAP (see Table 162-1) unless the patient has risk factors for MDR organisms (see Box 162-2). In the latter case, the authors recommend following the therapeutic recommendations for late-onset HAP (Figure 162-1 and Table 162-2) and obtaining samples for culture and a cytologic specimen representative of the lower airway to guide therapy.
Pneumonia
Community-Acquired Bacterial Pneumonia
Hospital-Acquired Bacterial Pneumonia
Species
Antimicrobial Recommendations
Dog
First-generation cephalosporin and second- or third-generation cephalosporin
or
Carbapenem
or
Amikacin and ticarcillin/clavulanate or amoxicillin/sulbactam
Cat
Same recommendations as for dogs; however, consider addition of enrofloxacin or doxycycline for treatment of Mycoplasma infection
Aspiration Injury
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine

