Chapter 30 Pleural Space Disease
PLEURAL SPACE
Physiologic fluid flux in the pleural space is governed by Starling’s law (Box 30-1). Hydrostatic pressure favors fluid accumulation within the pleural cavity (where pressure is subatmospheric), and the parietal pleura (systemic circulation) has greater filtration capacity than the visceral pleura (pulmonary circulation). However, oncotic pressure favors reabsorption of fluid from both pleura because the colloid osmotic pressure of the pleural space is 3.2 cm H2O in dogs (compared with 24.5 to 27 cm H2O in the vascular space). The visceral pleura assumes a larger role in determining the net pressure and favors reabsorption of fluid from the pleural space, where a greater vascular supply and lower hydrostatic pressure exist. Pleural lymphatic vessels are also an important component of fluid and blood reabsorption from the thorax.3
Box 30-1 Modified Starling’s Law Applied to the Pleural Cavity
Modified from Pleural effusion and diseases of the pleura, Vet Clin North Am Small Anim Pract 15:1069, 1985.
Net filtration = K{[(Pc parietal−Pc visceral)−Pif]−(πc−πif)}
CLINICAL EVALUATION
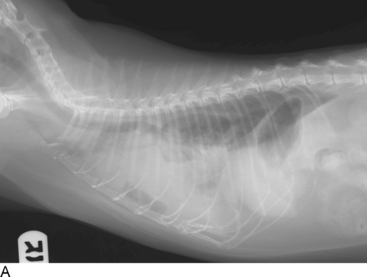
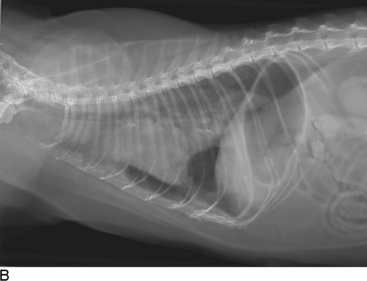
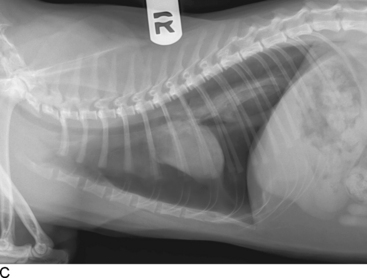
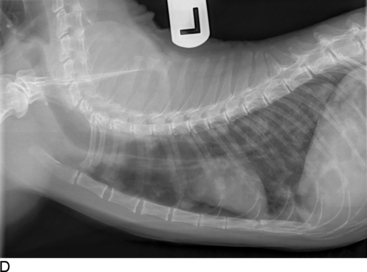
Thoracic radiographs are extremely helpful in diagnosing and quantifying pleural space disease and other intrathoracic pathology. Repeat radiographs after thoracentesis can be of diagnostic utility but were found to rarely be beneficial in providing additional diagnostic information in one human study.7 Routine radiographs after thoracentesis are considered unnecessary for stable patients in the absence of suspicion, clinical indication, or risk factors for complications (mostly pneumothorax)7,8 (Figure 30-1). Because the shape of the canine and feline chest is much different from that of humans, it is possible that dorsoventral radiographs in animals with pleural effusion (following thoracentesis) may still have improved diagnostic utility for evaluating the dorsal lung fields. Ultrasonographic examination is very helpful for rapid identification of pleural fluid in the emergency setting. In human medicine, indications for use of ultrasonographically guided thoracentesis include a small-volume effusion, inability to properly position the patient, failure of fluid to layer out on radiographs, and coagulopathy.9 “In veterinary medicine, ultrasound guidance is used routinely to confirm the ideal point of needle insertion for thoracentesis, mostly in patients with a small volume of effusion, fluid pockets or those at increased risk for complications.”
Thoracentesis is an invaluable diagnostic, and often therapeutic, tool (see Chapter 31, Thoracentesis). Its indications include (1) the presence of any undiagnosed pleural effusion and (2) therapeutic thoracentesis to relieve respiratory signs caused by large amounts of air or fluid. However, if the etiology of the effusion is known and the patient is not dyspneic, the procedure may be delayed and the clinical signs followed.14 Fluid analysis has great diagnostic utility in patients with pleural effusion of an undetermined etiology.14,15
PLEURAL EFFUSION
Pure Transudate and Modified Transudate
Transudative pleural effusion, or hydrothorax, is the result of variations in the Starling forces that govern pleural fluid flux (see Box 30-1). Pure transudates are characterized by a low total protein and total nucleated cell count (Table 30-1). Hydrothoraces generally develop secondary to decreased oncotic pressure within the vasculature and are commonly associated with hypoalbuminemia, although it also may be secondary to an increased hydrostatic pressure or neoplasia. Modified transudates are associated with an increased hydrostatic pressure (i.e., heart failure) or vascular permeability (e.g., vasculitis, lung lobe torsion, diaphragmatic hernia) causing leakage of a higher protein ultrafiltrate.16 However, in animals with chronic effusion, irritation of the pleura may cause an increased nucleated cell count and water can be reabsorbed in excess of protein and cells.6 Translocation of abdominal effusion, neoplastic effusion, and chylothorax are other causes of transudates.
Table 30-1 Fluid Type and Characteristics16
| Fluid Type | Fluid Characteristics |
|---|---|
| Pure transudate | TP <2.5 g/dl TNCC <1500/μl |
| Modified transudate | TP 2.5 to 7.5 g/dl TNCC 1000 to 7000/μl |
| Exudate | TP >3.0 g/dl TNCC >7000/μl |
TP, Total protein; TNCC, total nucleated cell count.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree