Chapter 164 A wide variety of diseases result in accumulation of fluid in the pleural space and cause respiratory compromise as expansion of the lungs is diminished. The underlying cause generally determines the prognosis and management. But first, the condition must be recognized in the patient and a sample of pleural fluid obtained for analysis. At that point, the pleural effusion can be classified into one of several primary categories (Table 164-1) based on the gross appearance, protein content, specific gravity, total nucleated cell count, and cytologic characteristics of the cells. Additionally, aerobic and anaerobic cultures may be indicated. Analysis of biochemical characteristics of the fluid, especially the triglyceride and cholesterol levels, is indicated if the effusion is suspected to be chylous (these values should be compared with serum levels). By identifying the type of effusion present, potential causes and the differential diagnoses for the effusion can be generated (Box 164-1). TABLE 164-1 Characterization of Pleural Effusions
Pleural Effusion
Overview of Pleural Effusions in Dogs and Cats
Fluid Type
Total Protein
Total Nucleated Cell Count
Pure transudate
<2.5 mg/dl
<1500/µl
Modified transudate
2.5-7.5 mg/dl
1000-7000/µl
Exudate
>3.0 mg/dl
>7000/µl
Initial Management of Pleural Effusion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


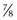 -inch butterfly needle is sufficient for most cats and small dogs. The needle tip should be placed just cranial to the rib to avoid intercostal blood vessels and nerves that are located caudally and should be inserted gently into the thorax perpendicular to the chest wall with the bevel of the needle pointing up while the hub of the needle and extension tubing are carefully observed for any signs of fluid. In cats with pleural effusion caused by heart failure, care must be taken to avoid puncturing a dilated left auricle, which often extends toward the left thoracic wall. Once the pleural space has been punctured, the needle can be directed ventrally so that the needle is almost parallel to the chest wall.
-inch butterfly needle is sufficient for most cats and small dogs. The needle tip should be placed just cranial to the rib to avoid intercostal blood vessels and nerves that are located caudally and should be inserted gently into the thorax perpendicular to the chest wall with the bevel of the needle pointing up while the hub of the needle and extension tubing are carefully observed for any signs of fluid. In cats with pleural effusion caused by heart failure, care must be taken to avoid puncturing a dilated left auricle, which often extends toward the left thoracic wall. Once the pleural space has been punctured, the needle can be directed ventrally so that the needle is almost parallel to the chest wall.