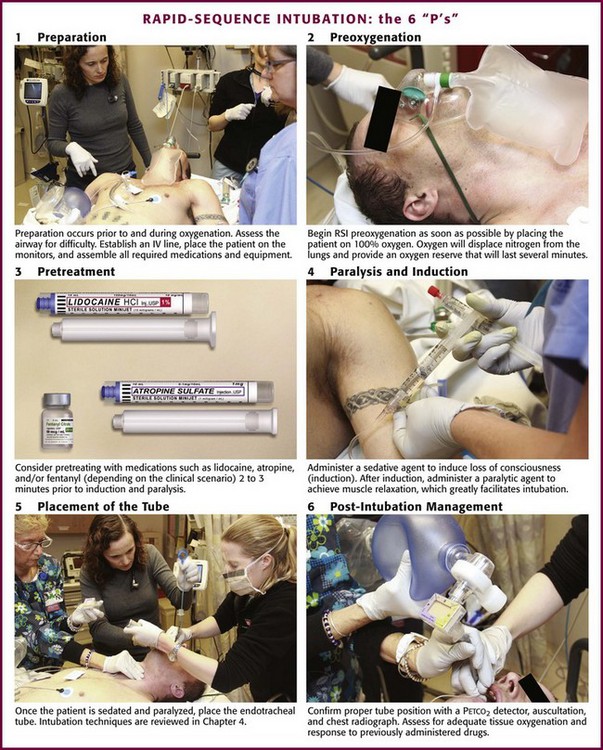
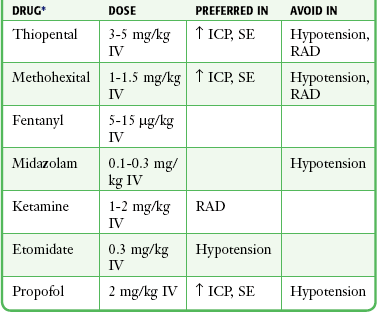
Chapter 5 In 1979, Taryle and coworkers1 reported that complications occurred in more than half the patients intubated in a university hospital ED. They called for improved house officer training in ET intubation, including “more liberal use of the procedures and agents used in the operating room (OR), including sedatives and muscle relaxers.”1 Since this report, the use of neuromuscular blockade and rapid-sequence intubation (RSI) has become the standard for emergency medicine practice.2,3 In addition to RSI, emergency physicians now use airway devices such as videoscopes and flexible fiberoptic bronchoscopes to manage difficult and complex airways. The sequential process for quickly intubating a patient in an emergency situation is referred to as rapid-sequence intubation. The steps in performing RSI are often described by the six “P’s”: preparation, preoxygenation, pretreatment and induction, paralysis, placement of the tube, and postintubation management (Fig. 5-1). This sequential technique of rapidly inducing unconsciousness (induction) combined with muscular paralysis and optimal conditions for intubation has gained broad acceptance among ED clinicians. Obviously, many patients do not afford the clinician the time or opportunity to comply with the ideal scenario of trachael intubation described in this chapter. RSI, as described in this chapter, is the ideal method of emergency airway management for intubations not anticipated to be difficult. Figure 5-1 The 6 “P’s” of rapid sequence intubation. IV, intravenous; RSI, rapid-sequence intubation. Begin RSI preoxygenation as soon as possible by administering 100% oxygen. The intent is to displace nitrogen from the lungs and replace it with an oxygen reserve that will last several minutes. Under optimal conditions, breathing 100% oxygen for 3 minutes has been demonstrated to maintain acceptable oxygen saturation for up to 8 minutes in previously healthy apneic individuals.4 Another method is to give four maximal breaths of 100% oxygen from a face mask, which can also maintain acceptable saturation for 6 minutes.4 Comparable results may not be expected in the ED setting, though, because of differences in the underlying health and cooperation of the patient population. Paralysis and induction involve the induction of a state of unconsciousness with a sedative agent, followed immediately by muscle paralysis. A protocol for ED-based RSI is summarized in Box 5-1. ET intubation and RSI have also expanded beyond the ED into the prehospital setting. Prehospital RSI protocols use a sedative plus a paralytic for patients not in cardiac arrest, with success rates as high as 92% to 98% being demonstrated.5–9 As in the ED setting, without a full complement of medications, prehospital intubation becomes significantly more difficult, and success rates drop to approximately 60%.10 Rates of misplaced ET tubes and complications by paramedics may be much higher than previously reported.11,12 Recent studies indicate that outcomes may be worse for patients with traumatic brain injury intubated in the prehospital setting than in the ED.13 For these reasons many prehospital systems have moved away from the use of RSI. The technique for proper ET tube placement is discussed in Chapter 2.1–3 Postintubation monitoring should assess for proper tube placement, adequate tissue oxygenation, and response to previously administered drugs. After laryngoscopy, the clinician should ensure ongoing analgesic and anxiolytic therapy. In addition to the ubiquitous sinus tachycardia, a number of dysrhythmias have been reported after intubation. They are primarily ventricular in origin and include ectopic beats, bigeminy, and short runs of ventricular tachycardia. Bradyarrhythmias have been reported uncommonly. Electrocardiographic changes suggestive of ischemia have been documented, particularly in patients with dramatic increases in blood pressure.14,15 In 1977, Fox and colleagues16 reported two patients, both of whom deteriorated after induction of anesthesia and orotracheal intubation. This report has been widely quoted as evidence that the pressor response should be prevented. No studies have reported comparative data, and none have established a direct relationship between the response and subsequent clinical deterioration in a large patient population. It is also unclear whether attenuation of the pressor response will prevent dysrhythmias or electrocardiographic evidence of ischemia, although it is prudent to avoid sudden increases in blood pressure in unstable patients with acute cardiac or vascular disease. Multiple studies have evaluated procedures to pharmacologically block the pressor response. Lidocaine has been the most extensively evaluated, but the results of these studies are inconclusive.17–23 No standards are universally accepted. Although it appears that administration of lidocaine, 1.5 to 2 mg/kg intravenously before intubation, may blunt the response, it is not clear that the reductions reported (10 to 15 mm Hg and 20 beats/min) are of any clinical significance. Other drugs, including thiopental, sodium nitroprusside, labetalol, nitroglycerin, verapamil, nifedipine, clonidine, fentanyl, sufentanil, etomidate, and magnesium, have shown variable responses.24–33 Of these drugs, fentanyl may be the most effective. It completely suppresses the pressor response at large anesthetic doses of 50 µg/kg,34–36 but considerably smaller doses may be effective. Two studies have shown marked suppression of the pressor response at doses of 5 to 6 µg/kg, although in both studies patients also received 5 mg/kg of thiopental.18,37 Fentanyl has blunted the pressor response when administered in conjunction with etomidate.32 The 1998 SHRED (Sedatives and Hemodynamics during Rapid-Sequence Intubation in the Emergency Department) study evaluated the pressor response to RSI and compared thiopental, fentanyl, and midazolam as induction agents.38 Midazolam, which is associated with the poorest intubating conditions and the most attempts required for intubation, showed a mean increase in heart rate of 17 beats/min. Thiopental, probably because of its direct myocardial depressant and venodilatory effects, decreased mean arterial pressure by about 40 mm Hg. Fentanyl recipients maintained a relatively neutral hemodynamic profile during intubation. Use of paralytics during intubation did not appear to alter the hemodynamic response associated with each sedative agent. One study isolated the effect of laryngoscopy from the effect of tube passage into the trachea. Adachi and colleagues39 found that pretreatment with 2 µg/kg of fentanyl could blunt the hemodynamic effects of tracheal tube passage, but not the hemodynamic effects of laryngoscopy. It is important to note that even studies demonstrating blunting of the pressor response failed to show that this provided any real benefit to patients. It is likely that the pressor response is innocuous in the vast majority of patients, but it may be exaggerated and potentially harmful in those with preexisting hypertension, cardiovascular disease,40 or other vascular comorbid conditions, in whom sudden changes in hemodynamics may be detrimental.41 The pressor response may contribute to the rise in ICP that follows laryngoscopy, and it is potentially harmful in patients with intracranial pathology. Administration of lidocaine or fentanyl to blunt the pressor response may be appropriate in these subsets of patients, but no universal standards exist. Physical stimulation of the respiratory tract by maneuvers such as laryngoscopy, tracheal intubation, and ET suctioning is commonly associated with a brief rise in ICP. The exact mechanism responsible for this rise is unknown. One potential mechanism is the coughing and gagging that frequently follow manipulation of the upper airway and subsequent transmission of intrathoracic pressure to the cerebral circulation. An alternative explanation is the release of catecholamine that accompanies laryngoscopy, which causes a rise in mean arterial pressure and cerebral perfusion pressure. A small rise in ICP has been reported after the administration of succinylcholine. The value of pretreatment with defasciculating doses of neuromuscular blockers (NMBs) to prevent rises in ICP is unknown.42 Good evidence suggests that deep general anesthesia prevents the rise in ICP associated with intubation. Depending on the drug used, anesthesia may compromise cardiovascular performance and critically reduce cerebral blood flow.43–45 Consequently, the ideal anesthetic agents to facilitate intubation of patients with acute intracranial pathology may be those that have minimal effects on cardiovascular performance, such as etomidate or fentanyl. Etomidate has been demonstrated to prevent changes in both cerebral perfusion pressure and ICP after tracheal intubation of patients with space-occupying intracranial lesions.46 At the present time the clinical consequences of intubation-induced physiologic changes are not thoroughly understood. The role of drugs in preventing these changes is equally unclear. Despite this lack of data, it may be intuitively reasonable to attempt to protect patients at theoretical risk. The approach outlined in Box 5-2 is recommended. After premedication, a sedative agent is used to induce loss of consciousness. A number of diverse drugs are available in the ED to induce unconsciousness before intubation, including barbiturates, benzodiazepines, etomidate, ketamine, opiates, and propofol. The choice of a particular induction agent depends on the experience and training of the clinician, the patient’s clinical status, drug characteristics, and institutional protocols (Box 5-3). Considerable evidence indicates that the sedative agent selected influences the quality of intubation conditions and the rapidity of their attainment. These effects persist even when paralytic agents are used. Commonly used drugs and their doses are summarized in Table 5-1. TABLE 5-1 Recommended Anesthetic Dosing for Rapid-Sequence Intubation and Clinical Considerations ICP, intracranial pressure; IV, intravenously; RAD, reactive airway disease; SE, status epilepticus. *Any of these can drugs be used before the administration of a neuromuscular blocking agent to induce anesthesia (see text). The barbiturates rapidly cross the blood-brain barrier and induce unconsciousness in less than a minute. They are rapidly redistributed and then ultimately degraded in the liver. After a single IV dose of thiopental, anesthesia lasts 5 to 10 minutes, as compared with 4 to 6 minutes for methohexital.47,48 The recommended dose of thiopental is 3 to 5 mg/kg intravenously administered over a 60-second period. Methohexital is dosed at 1 to 1.5 mg/kg intravenously over a 30- to 60-second period. Advantages of barbiturates as induction agents include their high potency, rapid onset, and short duration of action. They reduce cerebral metabolism, oxygen consumption, cerebral blood flow, and ICP.49,50 For this reason, thiopental is considered the agent of choice for induction of anesthesia and maintenance of long-term anesthesia in patients with elevated ICP. Short-term use of barbiturates during RSI has not been shown to have a protective effect on the CNS. Barbiturate use in hypovolemic patients may lead to systemic hypotension and impaired cerebral perfusion pressure, which may offset any cerebral-protective characteristics.38 Some evidence indicates that for ED RSI, thiopental may produce the best intubating conditions when used in conjunction with succinylcholine.51 The most significant complication of barbiturate therapy is depression of the vasomotor center and myocardial contractility, which can lead to significant hypotension. One study showed the average decrease in mean arterial pressure during RSI with thiopental to be 40 mm Hg.38 This is particularly pronounced in the presence of hypovolemia or cardiovascular disease. Etomidate is an ultrashort-acting nonbarbiturate hypnotic agent that has been used successfully as an anesthesia induction agent in Europe since the mid-1970s and in the United States since 1983. A significant benefit of etomidate in the emergency setting is its lack of cardiodepressant effects.52,53 Several case series have now demonstrated its safety and effective use in ED RSI.54–58 Extensive experience with this agent now exists in both pediatric59 and adult patients, and it is an agent of choice for most ED intubations. Etomidate is a carboxylated imidazole that is both water and lipid soluble. The drug reaches peak brain concentrations within 1 minute of IV infusion60 and induces unconsciousness within 30 seconds of administration. Its effects last less than 10 minutes after a single bolus dose.10 Redistribution of the drug is quite rapid, which accounts for its short duration of action. Etomidate is rapidly hydrolyzed in the liver and plasma and forms an inactive metabolite excreted primarily in urine.60 The recommended dose is 0.3 mg/kg via rapid IV bolus. There is virtually no accumulation of the drug, and anesthesia may be maintained through repeated doses; however, etomidate should not be used as an infusion or in repeated bolus doses for maintenance of sedation after intubation in the ED.61 Etomidate acts on the CNS by stimulating γ-aminobutyric acid (GABA) receptors and depressing the reticular activating system. It produces electroencephalographic changes similar to those produced by barbiturates as patients pass rapidly through light to deep levels of surgical anesthesia. Because etomidate has no analgesic activity,60 it should be used in conjunction with a parenteral analgesic when painful conditions are being treated, although it is most commonly used as a sole induction agent for intubation. Etomidate decreases cerebral oxygen consumption, cerebral blood flow, and ICP but appears to have minimal effects on cerebral perfusion pressure.46 Most importantly in the ED setting, etomidate is characterized by hemodynamic stability without significant changes in mean arterial pressure,53,54,57 although a slightly increased heart rate may be observed.61 Etomidate is suggested for induction of patients with significant cardiovascular disease requiring RSI. The hemodynamic stability that it provides and the absence of induced hypertension make it preferable to other sedatives. This hemodynamic stability persists even in patients with preexisting hypotension.62 The most common immediate side effects of etomidate are pain on injection, nausea, vomiting, and myoclonic jerks.63 Pain on injection is reported in up to two thirds of patients. Use of a large vein, simultaneous saline infusion, and opioid premedication can reduce the discomfort in appropriate situations.64 Myoclonic activity has been reported in about one third of cases and is believed to be caused by disinhibition of subcortical activity rather than CNS stimulation and does not represent seizure activity.60 This sometimes dramatic effect can be avoided through the use of NMBs and is rarely seen in the ED, where paralytic agents are regularly used with RSI. No treatment of myoclonus is usually necessary, and it is generally of no clinical significance. If persistent, an intravenous benzodiazepine may be administered. Etomidate need not be avoided in patients with seizure disorders, status epilepticus, head injury, or stroke. Some degree of altered adrenal function has been demonstrated even after a single dose of etomidate.65,66 The true clinical effect is unknown, but because the alteration in adrenal function appears to persist for 12 to 24 hours, there is theoretical concern about potential clinical consequences.10,67,68 Etomidate is a reversible inhibitor of 11β-hydroxylase, the enzyme that converts 11-deoxycortisol to cortisol. Although cortisol levels do not fall below the normal physiologic range, even a single induction dose of etomidate causes a measurable decrease in the level of circulating cortisol that occurs in response to the administration of exogenous adrenocorticotropic hormone (ACTH). Despite concerns regarding the safety of etomidate in the specific setting of adrenal insufficiency related to sepsis, no well-designed, prospective trial has shown adverse effects from a single dose of etomidate used for intubation in patients with sepsis or septic shock. Overall, however, the literature on this subject provides conflicting results.69–86 When comparing etomidate with ketamine, a multicenter randomized trial of critically ill patients requiring emergency intubation found no significant difference in organ failure score, 28-day mortality, or intubating conditions between patients given etomidate for induction and those given ketamine.83 No serious, drug-related adverse events were reported with either medication. Even though adrenal insufficiency occurred at a higher rate in the etomidate group (86%), it also developed in approximately 48% of patients receiving ketamine. Although historically no individual study is sufficiently powered to detect differences in mortality from the adrenal effects of etomidate, a systematic review of 20 studies in which etomidate was given in a bolus dose as part of induction for tracheal intubation found that etomidate does not have a significant proven effect on mortality.84 Declines in serum cortisol concentration were more prevalent in etomidate recipients than in those who did not receive etomidate in the large majority of studies, but the effect did not persist beyond 5 hours. When intubating a critically ill patient with possible adrenal insufficiency, the clinician must weigh the theoretical risk of cortisol suppression against the hemodynamic instability that may be caused by alternative induction agents. Overall, the mixed results in the current literature do not justify recommendations to avoid using etomidate for induction in patients with sepsis. Pending more definitive studies and subject to change as additional evidence is forthcoming, etomidate is an acceptable induction agent for patients with severe sepsis.87 Ketamine is a water- and lipid-soluble drug that rapidly penetrates into the CNS. Like the barbiturates, ketamine accumulates rapidly and then undergoes redistribution with subsequent degradation in the liver.88 The recommended dose of ketamine before intubation is 1 to 2 mg/kg administered intravenously over a 1-minute period. Anesthesia occurs within 1 minute of completing the infusion and lasts 5 to 10 minutes. A smaller additional dose (0.5 to 1 mg/kg) may be given 5 minutes after the initial dose if needed to maintain anesthesia. Simultaneous administration of succinylcholine and midazolam is recommended to provide adequate muscle relaxation and to decrease the incidence of postanesthesia emergence reactions, respectively. The intramuscular dose for intubation has not been well studied, but a suggested dose is 4 to 5 mg/kg. Onset of action occurs within 2 to 3 minutes. Because of its good vascularity, the anterior thigh muscle is theoretically the preferred site for administration. Unlike other anesthetic agents that depress the reticular activating system, ketamine acts by interrupting association pathways between the thalamocortical and limbic systems. Characteristically, the eyes remain open, and patients exhibit spontaneous, though not purposeful movements. Increases in blood pressure, heart rate, cardiac output, and myocardial oxygen consumption are seen, most likely mediated through the CNS. In vitro studies indicate that ketamine is a myocardial depressant, but the CNS-mediated pressor effects generally mask the direct cardiac effects,89,90 thus making it potentially useful in patients with hemorrhagic shock or hypotension. Respirations are initially rapid and shallow after ketamine administration, but they soon return to normal. In one study, eight patients with traumatic brain injury and elevated ICP who were sedated with propofol were given different doses of ketamine (1.5, 3, or 5 mg/kg).91 Ketamine did not alter cerebral hemodynamics at any time. In another study, researchers randomly assigned 25 patients with traumatic brain injury to sedation with either ketamine and midazolam or sufentanil and midazolam.85 Other studies suggest that ketamine does not interfere with cerebral metabolism or increase cerebral oxygen consumption and does not reduce regional glucose metabolism.86 Ketamine can also offset any decrease in mean arterial pressure caused by fentanyl, a drug commonly used as part of RSI in patients with a head injury.92 The most promising use of ketamine as an intubation adjunct has been in the setting of acute bronchospastic disease. Ketamine relaxes bronchial smooth muscle either directly through enhancement of sympathomimetic effects or indirectly through inhibition of vagal effects. Ketamine also increases bronchial secretions and may decrease the incidence of mucous plugging, which is commonly seen in decompensating asthmatic patients.93 Clinical reports have demonstrated a reduction in airway resistance and an increase in pulmonary compliance within minutes of ketamine administration.88,94 Bronchospastic patients struggling to breathe and unable to tolerate oxygen masks or bronchodilators because of hypoxic encephalopathy will continue to breath deeply and rapidly with ketamine anesthesia, thereby allowing the maximum delivery of oxygen before a more elective intubation (Fig. 5-2).
Pharmacologic Adjuncts to Intubation
Overview of Rapid-Sequence Intubation
Pretreatment: preventing the Complications of Intubation
The Pressor Response
Intracranial Hypertension
Induction Agents
Barbiturates: Thiopental and Methohexital
Etomidate
Ketamine
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Pharmacologic Adjuncts to Intubation