CHAPTER 9 Peritoneal Fluid
Peritoneal fluid evaluation is a diagnostically useful adjunct to history, physical examination, and routine CBC and biochemical evaluation of abdominal disease in both the horse and the foal. Abnormalities in peritoneal fluid may be associated with a variety of disorders affecting the equine, including colic, peritonitis, traumatic injury, and neoplasia. Serial evaluation of peritoneal fluid is useful in discerning the need for surgical intervention, monitoring progression of disease and response to therapy, and determination of prognosis.
Peritoneal fluid can be readily collected via abdominocentesis in the minimally restrained, standing horse by the veterinarian in the field. Abdominocentesis may also be performed in laterally recumbent horses. This technique is most commonly employed in fluid collection from foals. Various collection techniques have been thoroughly detailed elsewhere.1–3 Equipment requirements are minimal and include clippers, surgical scrub, local anesthetic agent, sterile gloves, scalpel, teat cannula, gauze sponges, and collection tubes. Use of aseptic technique is essential. Tranquilizers may be required if the horse is intractable. Analgesics may be required if colic causes severe abdominal pain, so the operator can safely attempt fluid collection. Unless drainage of large volumes of abdominal effusion is medically indicated, a specimen of 3 to 5 ml is generally adequate for complete fluid analysis. The specimen may be divided among collection tubes containing transport media suitable for microbiologic culture, sterile clot tubes or heparin-containing anticoagulant tubes suitable for biochemical analysis, and EDTA anticoagulant tubes for cell counts, preservation of cell morphology, and cytologic examination.
Potential Complications of Abdominocentesis
Blood Contamination
Blood in the abdominocentesis specimen may be the result of peripheral blood contamination such as occurs with superficial hemorrhage from skin incision, perforation of abdominal muscles when the cannula or needle strays from the midline, perforation of a blood vessel on the serosal surface, or perforation of an abdominal organ (especially the spleen). Conversely, hemorrhagic diapedesis from a lesion in the peritoneal cavity or hemoperitoneum represent causes of true hemorrhagic effusions. Discerning contamination from true hemorrhage is important for accurate interpretation of fluid analysis results. A variety of gross and microscopic findings may aid in distinction of these processes. During specimen collection, if the peritoneal fluid flowing from the cannula or needle is initially free of hemorrhage and then becomes bloody, contamination at collection is obvious. Similarly, if the peritoneal fluid is initially bloody and then clears of hemorrhage as it is collected, contamination is likely. Such observations should be noted on the animal’s case record and/or on the clinical pathology request form, as appropriate. When the specimen is uniformly bloody or discolored throughout collection, and at different sites, hemorrhagic diapedesis or hemoperitoneum is likely. Estimation of peritoneal fluid quantity and comparison of fluid and whole blood packed cell volume (PCV) may assist in differentiating hemorrhage from contamination. Large volumes of grossly bloody peritoneal fluid are more compatible with true intraperitoneal hemorrhage; a high PCV is useful to confirm this suspicion. Further microscopic differentiation of these possibilities are discussed later (see section on hemorrhagic effusions).
Peripheral blood contamination can be minimized by using several precautions during specimen collection. When one is selecting an abdominocentesis site, obvious superficial veins must be avoided. Nevertheless, cutaneous incision frequently causes minor hemorrhage. Consequently, the stab wound should be blotted with a sterile gauze sponge immediately before the cannula or needle is inserted through the abdominal wall. Whenever possible, a sterile, blunt-tipped teat cannula is preferred over needles for specimen collection, because it minimizes the risk of vessel or organ laceration. Walking or rocking the animal to try to increase the amount of peritoneal fluid in the ventral abdominal cavity during abdominocentesis is not recommended, especially when a needle is used for sample collection, because of the increased risk of intestinal laceration.4
Enterocentesis
Occasionally during abdominocentesis, the cannula or needle accidentally perforates the intestine. Contamination of peritoneal fluid with intestinal contents is frequently evident grossly. A green to brown fluid color, fermentative odor, and flocculent appearance suggest penetration of the gastrointestinal tract, particularly in a horse not exhibiting clinical signs consistent with rupture. Enterocentesis fluid may be grossly normal in appearance, so microscopic examination is essential to rule out of this potential complication.5
The reported frequency of accidental enterocentesis has varied from 2% to 5%, without any significant clinical sequelae. Another author attributed severe complications to accidental enterocentesis in three animals (0.4% of abdominocenteses over a 2-year period).4 Complications most frequently occurred in horses with preexisting gastrointestinal compromise secondary to a distended viscus.
Despite the infrequency of serious clinical complications following enterocentesis, one study demonstrated (by serial abdominocenteses) that peritonitis was evident within 4 hours.5 Total nucleated cell counts within the peritoneal fluid peaked 2 days after enterocentesis, with a mean nucleated cell count of 113,333/μl and maximum observed count of 540,000/μl. Neutrophils comprised the overwhelming majority of these cells and commonly showed toxic change; however, bacteria were not observed. The total nucleated cell count had decreased considerably by day 4 (when the study ended), with a mean nucleated cell count of 8650/μl and a maximum observed count of 25,400/μl. Nevertheless, all nine horses were clinically normal throughout the study, with the exception of one animal that was febrile on day 1. Peripheral blood total and differential leukocyte counts did not alter significantly at any stage.
Effects of Repeat Abdominocentesis or Laparotomy
In some cases, abdominocentesis may need to be repeated on several occasions to monitor changes in peritoneal fluid, and the changes observed may be critical in determining the need for surgical intervention. Serial, uncomplicated abdominocenteses in normal horses cause little change in peritoneal fluid composition.8,9 The total nucleated cell count and total protein concentration may increase slightly but do not typically exceed reference values.5 Differential cell counts also remain unchanged. Cell morphology remains normal, possibly with some hypersegmentation of neutrophils and some leukophagocytosis. With mild blood contamination, a small increase in red blood cell (RBC) numbers may be noted, with evidence of erythrophagocytosis.
Peritoneal fluid analysis may be useful in the postceliotomy patient for early detection of complications such as hemorrhage or sepsis and to monitor for resolution of underlying disease. Postoperative changes in peritoneal fluid values have been well documented10–12 and may be difficult to discern from pathologic alterations when conventional reference ranges are used. In such circumstances, use of specialized reference ranges10,11 is necessary for meaningful interpretation of fluid analysis results.
Specimen-Handling Considerations
Fluid should be collected into an EDTA tube for cell counts, cytologic examination, and (refractive index) total protein measurement. With small sample size (EDTA tubes less than one-quarter filled), erroneous results for fluid protein levels and cell counts may be obtained (see section on biochemical examination later in this chapter).13
Gross Fluid Examination
Gross visual inspection of fluid consists of noting fluid volume, color, turbidity, and odor. Normal peritoneal fluid is of small volume, clear to slightly turbid or opalescent, pale yellow to straw colored (depending on diet),14 and odorless. Much useful information may be gained by assessing the ease with which the fluid is obtained and by visually appraising the turbidity and color of the sample. A total nucleated cell count, cytologic examination, and total protein measurement should be performed on all specimens. Caution must be exercised to resist the temptation to limit fluid analysis simply to gross visualization, since grossly “normal-appearing” peritoneal fluid is sometimes observed with accidental enterocentesis,5 as well as with disease conditions such as ruptured bladder.15
Volume
There is little free peritoneal fluid in the abdominal cavity of a healthy horse, the quantity being only sufficient to ensure lubrication of the parietal and serosal mesothelial surfaces.16,17 Nevertheless, in 20 clinically normal horses examined at necropsy, about 100 to 300 ml of free peritoneal fluid was found.18 These estimates are probably below the actual amount of fluid bathing the mesothelium of the abdominal cavity, but they reflect the approximate quantity of fluid potentially available for sampling by abdominocentesis. In an unpublished study of six clinically normal horses with peritoneal fluid cell counts, cytologic findings, and total protein concentration within published reference values, 580 to 2050 ml of fluid was estimated to be present based on a dye dilution technique.19
It is important for the veterinarian to adopt a standard technique for abdominocentesis, since this permits subjective estimation of the volume of fluid within the abdominal cavity, based on the ease of collection and rate of flow. Over a 5- to 10-minute interval, 10 to 100 ml of peritoneal fluid may be collected from most normal horses, with 50 to 60 ml commonly collected in 10 minutes.6,14 However, collection of such large volumes is not necessary for routine analysis of peritoneal fluid. Usually 3 to 5 ml can be easily collected from most animals and is adequate for laboratory purposes.7 If abdominocentesis is nonproductive or if samples are bloody, additional attempts can be made in alternate locations remote from the initial abdominocentesis site. Several such attempts should be made before the abdominocentesis is considered to be a dry tap (no fluid collected despite several attempts at different locations). This may occur when:
Color and Clarity
When the specimen grossly resembles whole blood, estimation of specimen quantity, determination of fluid PCV, clotting times, and cytologic appearance may be useful in distinguishing contamination or splenic tap from hemoperitoneum. A small-volume specimen having a PCV similar to or greater than peripheral blood, without cytologic evidence of erythrophagia, is characteristic of a splenic tap.1,2 Contaminated specimens most often have a PCV significantly less than that of peripheral blood, and platelet clumps may be visualized microscopically. Increased fluid volume, failure of the specimen to clot, or presence of significant erythrophagia upon microscopic examination suggests true hemoperitoneum. Specimen color may aid in differentiating previous from recent/ongoing hemorrhage: a bright red color suggests recent or ongoing hemorrhage, while a reddish brown to brown color may be compatible with previous hemorrhage.
Reddish brown, port wine, or muddy effusions are also frequently associated with ischemic tissue injury/necrosis and suggest a poor prognosis.1,2 Presence of degenerative leukocyte changes (loss of nuclear segmentation, indistinct nuclear margins) with concurrent presence of bacterial organisms is compatible with devitalized bowel. Additionally, a brown or golden brown peritoneal fluid may rarely occur in peritoneal effusions associated with metastatic pigmented melanoma.
Dark green fluid color is compatible with the presence of free bile as a result of damage to the bile duct or rupture of the duodenum.1 Additionally, observation of bright orange bilirubin crystals or a positive Ictotest on the fluid specimen would support bile peritonitis. A bright green fluid color is more compatible with enterocentesis of the large colon or cecum. Mixed bacterial organisms and/or plant material would aid in the distinction of enterocentesis from bile peritonitis.
Cytologic Examination
A cell count, total protein determination, and cytologic evaluation can be performed in the laboratory. Such tests require little equipment and yield much useful information. The method by which smears are prepared in the laboratory for cytologic evaluation varies with the specimen’s cellularity and the equipment available. Fluids with a total nucleated cell count <5000/μl are most readily examined if smears are made after cells are concentrated by some means. In diagnostic laboratories, special cytocentrifuges are often used for this purpose. A 100-μl aliquot yields ideal cell morphology for cytospin examination of most fluid specimens (Figs. 9-1 and 9-2). Transudative, very-low-cellularity effusions may require up to 200 μl to improve cellularity for microscopic examination. Extremely high-cellularity, exudative effusions may require a reduced volume aliquot (25 to 50 μl) or specimen dilution to achieve cytospin preparations with ideal cell morphology and numbers.
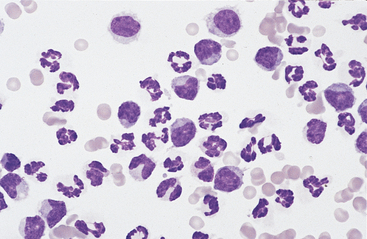
Fig. 9-1 Cytocentrifugation of normal equine abdominal fluid for cytologic examination.
Concentration of cellularity in this fashion yields the ideal cell density and morphology for microscopic examination of most fluid specimens. A 100-μl aliquot is sufficient for most samples. In this fluid, nondegenerate neutrophils are the predominant cell type. (Wright-Giemsa)
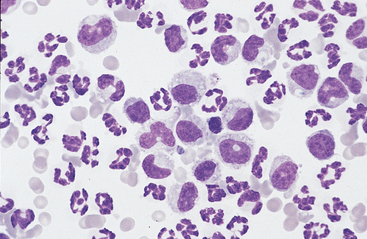
Fig. 9-2 Cytocentrifugation of normal equine abdominal fluid showing a greater percentage of large mononuclear cells than in Fig. 9-1.
A range of nuclear morphology is characteristic of this cell type. Nuclei may vary from round to oval to bean-shaped to lobulated. (100-μl aliquot, Wright-Giemsa)
In laboratories lacking a cytocentrifuge, specimens may be concentrated for cytologic examination with centrifugation of up to 10 ml of peritoneal fluid in a tube for about 5 minutes at 1000 to 1500 rpm. The resultant supernatant is removed and saved for total protein measurement. The sedimented cells are then gently resuspended in about 0.25 to 0.5 ml of peritoneal fluid and smears prepared, often using a line smear technique to concentrate cells at the leading edge of the smear (see Chapter 1). Romanowsky-type stains are usually used, such as Wright’s, May-Grünwald, Giemsa, or Diff-Quik. Fluids with a total nucleated cell count of 5000 to 10,000/μl may be prepared from centrifuged or uncentrifuged specimens, depending on the operator’s preference. (Line smears from centrifuged sediments yield more cellular and therefore more easily scanned smears in these situations.) When the total nucleated cell count is >10,000/μl and the fluid’s turbidity is therefore greater than normal, direct smears of uncentrifuged specimens are usually satisfactory.
Cell Counts
Total nucleated cell counts of peritoneal fluid are performed as for a blood sample. This can vary from manual dilution with microscopic enumeration to the use of automated cell counters. Reference values for peritoneal fluid of mature aged horses and of foals are summarized in Table 9-1. Small volume specimens (EDTA tube less than one-quarter filled) may be sufficiently diluted to mildly decrease cell counts.13
TABLE 9-1 Reference Values for Cell Counts and Refractometer Total Protein and Specific Gravity in Equine Peritoneal Fluid
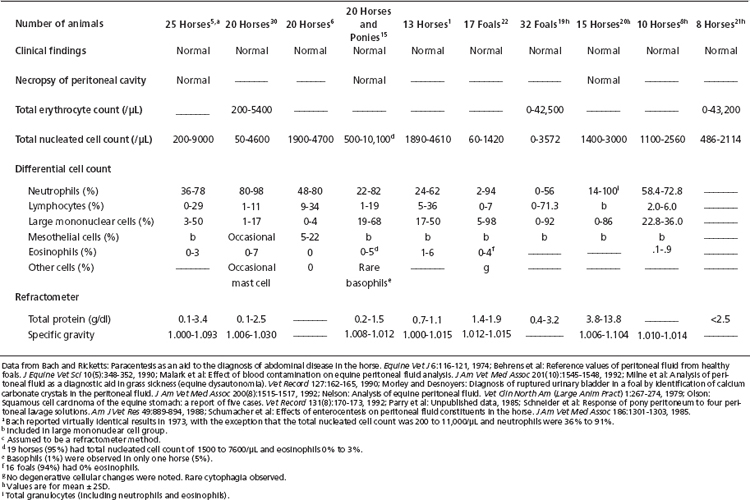
As a generalization, the total nucleated cell count is typically less than 10,000/μl (10.0 × 109/L) and often below 5000/μl (5.0 × 109/L) in mature or aged horses.9,21,22 Foals appear to have appreciably lower values.20,23 When a microhematocrit centrifuge is used to sediment cells from peritoneal fluid so that total protein content can be measured on the supernatant, the packed nucleated cell volume is less than 1%.6
Similarly, there is a negligible packed RBC volume. Normal peritoneal fluid contains very few RBCs (see Table 9-1) and negligible erythrophagocytosis. Erythrocyte counts are not often performed on peritoneal fluid unless automated techniques are employed that routinely include red cell number determination.
Reference values for peritoneal fluid cytologic composition are listed in Table 9-1. The predominant cells usually are neutrophils, followed by large mononuclear cells and then lymphocytes. Eosinophils are uncommon. Basophils and mast cells are rarely observed.
Cell Morphology
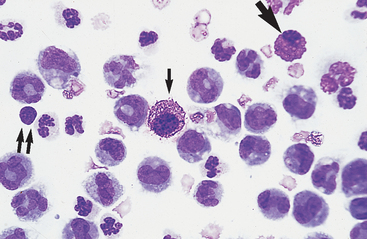
Neutrophils: Neutrophils entering the peritoneal cavity (like neutrophils entering other body cavities or tissues) do not return to the bloodstream. Consequently, neutrophil aging and death are normal events. Aged neutrophils are often moderately hypersegmented to pyknotic cells14,16,18 (Fig. 9-3). Leukophagocytosis of senescent neutrophils by macrophages (Fig. 9-3) may be observed in peritoneal fluid collected from clinically healthy animals.6,14,16,18 Neutrophils in normal peritoneal fluid do not exhibit phagocytic activity per se.6,14,16,18
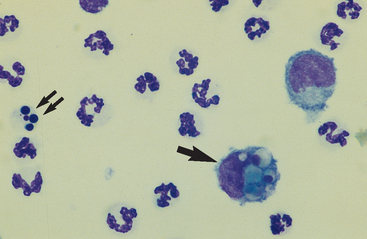
Fig. 9-3 Neutrophil morphology.
Neutrophils entering the peritoneal cavity do not return to the systemic circulation but age and die in the peritoneal fluid. Under normal circumstances, aging is noted as hypersegmentation of the nucleus and then pyknosis (the nucleus condenses and fragments) (double arrow). Such cells are removed (phagocytosed and digested) by macrophages (arrow). (Original magnification X950; May-Grunwald-Giemsa)
The presence of band neutrophils or more immature granulocytic cells suggests acute inflammation and mobilization of the neutrophil storage and maturation pools. Frequently, observation of immature granulocytic cells is associated with poor prognosis.24 The presence of degenerative changes (such as cell swelling, loss of nuclear segmentation, and indistinct nuclear margins)25 suggests a harsh peritoneal environment. This may occur secondary to the presence of bacterial cytotoxins or chemical irritants (bile or urine) within the abdominal cavity. Toxic changes (such as increased cytoplasmic basophilia, vacuolation, or Dohle bodies) may also be observed in septicemia or enterotoxemia. Such toxic change is considered to be “preexisting,” occurring during myelopoiesis, rather than subsequent to migration into the peritoneal cavity. These changes, accompanied by visualization of phagocytized bacterial organisms (see Figs. 9-13 to 9-15) are compatible with septic peritonitis.
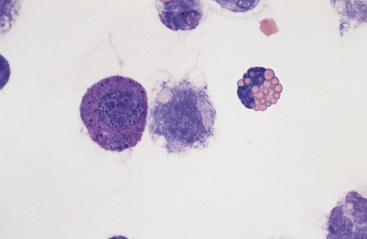
Large Mononuclear Cells: The category of large mononuclear cells encompasses nonreactive (tissue) macrophages of blood monocyte origin, reactive (tissue) macrophages, and mesothelial cells. Because these cells are often difficult to distinguish morphologically, they are commonly grouped together.7,16,18,26 They are also often referred to collectively as mononuclear phagocytes, since all are potentially phagocytic. All of these cells are large, usually with a moderate to high nucleus to cytoplasmic ratio and abundant, somewhat basophilic cytoplasm (Fig. 9-4). The nucleus is their most distinctive feature, but even that is not particularly characteristic, and subclassification of large mononuclear cells is quite subjective.26
Mesothelial cells usually have an oval nucleus with a finely reticular chromatin pattern. They occur in the highest proportions in low-cellularity, high-volume effusions (transudates). When individualized, a fine eosinophilic “corona” of glycocalyx may be evident along the cell margin. In normal peritoneal fluid, they frequently occur in sheets or rafts, are uniform in appearance, and polygonal to rhomboid in shape. Transudative effusions may be associated with mesothelial proliferation resulting from loss of contact inhibition subsequent to fluid separation of mesothelial surfaces, and tufts or balls of mesothelial cells may be observed. In exudative effusions, mesothelial cells become reactive or transformed and exhibit features suggesting increased proliferation, including increased cytoplasmic basophilia, multinucleation, prominent nucleoli, and mitotic activity25 (Fig. 9-5). Hyperplastic/dysplastic features can begin to mimic neoplasia in severe inflammatory conditions,1 and in rare instances, fine azurophilic cytoplasmic granulation has been reported.24 Such cells are seen in very small numbers in normal peritoneal fluid.6,14,16,18 Peritoneal fluid collected several hours after death often contains numerous sheets of exfoliated mesothelial cells, similar to those seen in some effusions (Fig. 9-6). Such a finding may thus be a postmortem artifact.
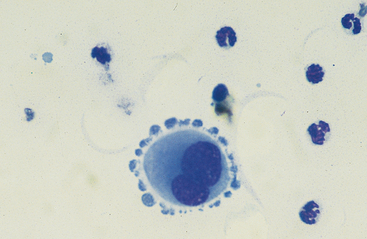
Fig. 9-5 Large reactive binucleate mesothelial cell in peritoneal fluid.
Note the apparent pseudopodia, which are an artifact of drying. (Original magnification X950; May-Grunwald-Giemsa)
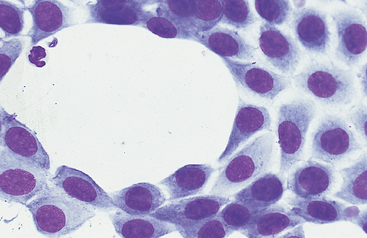
Fig. 9-6 Sheet of mesothelial cells in peritoneal fluid of horse with transudative effusion.
Sheets of cells may also be seen as a postmortem artifact in fluid collected several hours after death. (Original magnification X950; May-Grunwald-Giemsa)
The nonreactive macrophage (or monocytic cell) typically has an indented oval nucleus with a more homogeneous chromatin pattern. However, the nucleus of the latter cell can be quite pleomorphic, varying in shape from elongated, to round, to convoluted or lobulated. These cells are reported to constitute anywhere from approximately 5% to 80% of normal peritoneal nucleated cell differential counts.1 In acute inflammatory effusions, the relative percentage of monocytes/macrophages decreases with increasing neutrophil numbers. In more chronic effusions, mononuclear/macrophages are typically present in increased percentages and frequently exhibit reactive changes.25 Reactive cells tend to have more abundant, more basophilic cytoplasm. Reactive macrophages often have ruffled cytoplasmic margins, prominent cytoplasmic vacuoles, and/or inclusions (phagosomes). The latter may be unidentifiable debris or degenerating inflammatory cells or RBCs (see Figs. 9-3 and 9-4). Such cells, though readily apparent in normal peritoneal fluid, are present in small numbers.6,16,18 Cytophagia is rare in peritoneal fluid from foals.23
Lymphocytes: Lymphocytes in normal peritoneal fluid are usually small to medium-sized cells (see Fig. 9-4), similar to peripheral blood lymphocytes.14,16,18 These cells constitute only a small percentage of normal peritoneal fluid nucleated cells and recirculate into the bloodstream via the diaphragmatic lymphatic lacunae. An increased lymphocyte percentage may occur in chronic inflammatory conditions, parasitism, chyloperitoneum (particularly acutely), or neoplasia.
Lymphoblasts are not observed in normal peritoneal fluid. These cells have a densely staining delicate chromatin pattern, often with obvious nucleoli and intensely basophilic cytoplasm that may contain small to large vacuoles. Cytologic diagnosis of lymphoma is usually based on the presence of large numbers of such cells. Other considerations include marked inflammation24 or accidental aspiration of lymphoid tissue. Large granular lymphocytes have been observed in peritoneal fluid with both inflammatory disease24 and large granular lymphoma.27 Plasma cells may also be observed in low numbers in inflammatory fluid specimens and reflect chronic antigenic stimulation.16 Occasionally these cells may contain cytoplasmic vacuoles distended with immunoglobulin (Russell bodies).24
Other Cells: Eosinophils (Figs. 9-4, 9-7, and 9-8) and basophils have the same morphology as in a peripheral blood smear. Mast cells (Figs. 9-4 and 9-7) have the same morphology as in other tissue specimens and are an infrequent finding, even in inflammatory conditions. Increased eosinophil percentage (Figs. 9-8 and 9-9), possibly associated with a concurrent increase in basophils and mast cells, is inconsistently associated with intestinal parasitism or parasitic larval migration6,16,24,25 and may also accompany other septic or nonseptic causes of inflammation as well as neoplasia.25,28 There is often an accompanying neutrophilic exudate.
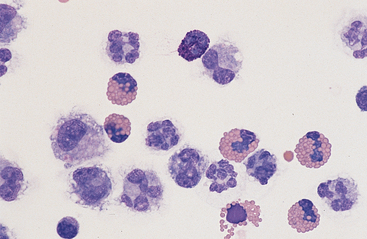
Fig. 9-8 Eosinophilia in abdominal fluid
Eosinophilia may be accompanied by the presence of basophils (center bottom). (Wright-Giemsa)
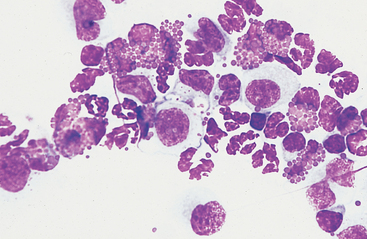
Fig. 9-9 Numerous eosinophils in peritoneal fluid of horse with parasitic hepatitis.
(Original magnification X950; May-Grunwald-Giemsa)
Neoplastic cells are occasionally identified in peritoneal fluid cytology. Neoplastic round cells (most commonly lymphoblasts) and epithelial cells (mesotheliomas and carcinomas) are those most commonly encountered.25 Criteria of malignancy include anisocytosis, anisokaryosis, variation in nucleus to cytoplasm ratio, increased cell size, nuclear gigantism, multinucleation, prominent/large/angular/multiple nucleoli, and increased/abnormal mitotic activity. Presence of concurrent inflammation may cloud distinction from reactive hyperplasia/dysplasia in some instances,25 necessitating further diagnostic investigation. Absence of identifiable neoplastic cells on peritoneal fluid cytology does not rule out neoplasia, since tumor cells do not consistently exfoliate into peritoneal fluid, even in disseminated disease.29,30
Biochemical Examination
Biochemical analysis of peritoneal fluid routinely involves determination of protein content, specific gravity, and fibrinogen concentration. A variety of additional biochemical parameters may be measured in unique diagnostic circumstances or in research applications. For total protein, specific gravity, and fibrinogen measurements, peritoneal fluid is usually collected in an EDTA tube (as for cytologic examination). When sample volume is small (EDTA tube less than one-quarter filled), fluid protein measurements, as determined by refractive index, may be artificially increased (solute effect of sodium or potassium EDTA), whereas biuret protein concentration results may be artificially decreased (dilutional effect).13 Although the artifactual effect is generally mild, it may reach diagnostic significance in distinguishing early, low-volume exudates from normal or borderline modified transudates from either pure transudates or mild exudates.
Total Protein Content
Total protein concentration is measured using a refractometer or by a biochemical method such as the biuret reaction (as for a blood sample). Refractive index is rapid, easy, and inexpensive; however, it is less accurate at low protein concentrations than biochemical methods. Reference values are given in Tables 9-1 and 9-2. As a generalization, the total protein concentration of normal peritoneal fluid is <2.5 g/dl (25 g/L), which is usually the lowest reading on the protein scale of most refractometers. Some authors report that any value >1.5 g/dl, as measured by the biuret reaction, indicates pathologic change.24
Specific Gravity
Because of the low total protein content of peritoneal fluid, specific gravity is commonly measured. Specific gravity is the density of a fluid as compared with distilled water (which has a value of 1.000). It is a reflection of the amount of dissolved solutes in the fluid, such as total protein, glucose, urea, and bilirubin. Reference values are given in Table 9-1. Total protein values are more commonly reported than specific gravity measurements.
Fibrinogen Content
Normal peritoneal fluid contains a negligible amount of fibrinogen and therefore does not clot. Using a heat precipitation method, fibrinogen concentrations <50 mg/dl (0.5 g/L) and <100 mg/dl (1 g/L) have been reported.18,31 These figures are probably overestimated because the heat precipitation method is not very sensitive to low fibrinogen concentrations.
Other Biochemical Tests
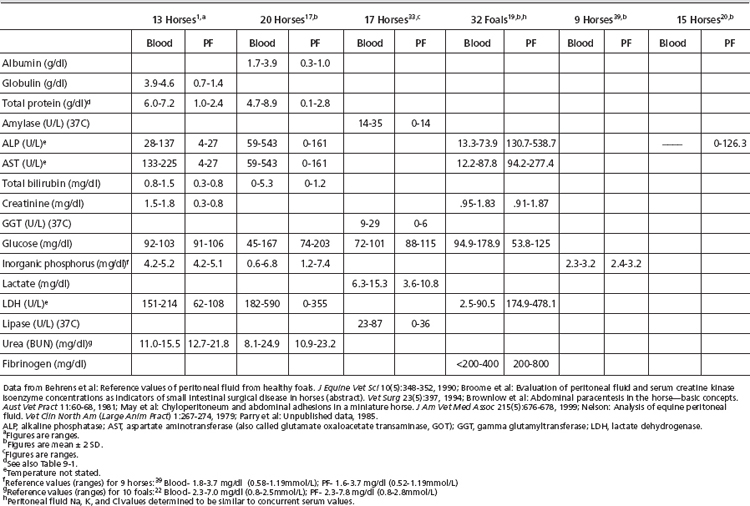
Reference values for paired blood and peritoneal fluid concentrations of the following analytes are presented in Table 9-2. Reference values for other biochemical tests have been published, including calcium, alanine aminotransferase (ALT), α-2-antiplasmin, antithrombin III (ATIII), creatine phosphokinase (CPK), chloride, fibrin(ogen) degradation products (FDP), pH, plasminogen, protein C, potassium, and sodium.1,18,20,32,33 The diagnostic merit of these assays has not been confirmed.
Water-soluble substances of low molecular weight, such as electrolytes (Na, K, Cl), glucose, phosphate, and urea, readily diffuse between the bloodstream and the peritoneal cavity. Consequently their concentrations in blood and peritoneal fluid are quite similar and well correlated.16,18 In contrast, substances of high molecular weight, such as enzymes, most proteins, and substances that are partly or primarily attached to carrier proteins, such as calcium and unconjugated bilirubin, are usually found in lower concentration in peritoneal fluid than in blood and take longer to equilibrate when there is an abrupt change in their concentration in either blood or peritoneal fluid. Consequently, serum and peritoneal fluid values of these substances are often poorly correlated.
Creatinine and Urea: Comparison of creatinine and urea concentration in the peritoneal fluid with that of serum values aids in the diagnosis of uroperitoneum. In health, peritoneal fluid values approximate serum values for both analytes. With acute uroperitoneum, concentrations of urea and creatinine in the peritoneal fluid will exceed those of serum, usually by a ratio of greater than 2:1.1,2,15,20,23 With time, equilibration of fluid and serum concentrations can occur, with diffusion of analytes from the fluid to the vascular space as well as with development of effusion secondary to chemical peritonitis. The former effect affects fluid urea concentrations more rapidly than creatinine concentrations because urea’s smaller molecular weight facilitates diffusion.
Glucose, Lactate, LDH, and pH: Water-soluble substances of low molecular weight, such as glucose and lactate, readily diffuse between the bloodstream and the peritoneal cavity. Peritoneal fluid glucose concentration in the healthy equine is reported to be similar to or slightly greater than that of serum.1,34,35 Increased anaerobic glycolysis by metabolically active cells (leukocytes or neoplastic cells) or bacterial organisms may decrease fluid glucose concentrations. Peritoneal fluid glucose concentrations <40 mg/dl or serum-to-fluid glucose differences of >50 mg/dl may be useful in predicting sepsis, particularly in horses with negative or pending fluid bacterial cultures.35
Additionally, increased anaerobic glycolysis would be expected to increase fluid lactate concentrations, thus lowering fluid pH. Detection of fluid lactate concentrations greater than those of paired blood specimens or fluid pH <7.3 lends further support to suspicion of sepsis.1,35
LDH catalyzes the oxidation of pyruvate to lactate during anaerobic glycolysis, and peritoneal fluid LDH activity would be anticipated to increase in parallel with decreasing fluid glucose concentration, increasing fluid lactate levels, and decreasing pH. In one study comparing peritoneal fluid LDH activities in horses with nonseptic and septic peritonitis, however, a significant difference was not detected between the groups.35 The authors hypothesized that tissue injury associated with celiotomy in the nonseptic peritonitis group may have contributed to elevated peritoneal fluid LDH activity. LDH activity may be of diagnostic utility in assessing likelihood of sepsis in the presurgical patient, however.
Cholesterol and Triglycerides: Chylous and pseudochylous effusions are grossly similar in appearance, and biochemical testing is frequently useful in distinguishing these fluids.36–39 Chylous effusions are characterized by a high triglyceride content, and fluid values will greatly exceed serum values. Conversely, fluid cholesterol levels remain low, less than paired serum values. In contrast, pseudochylous effusions typically have cholesterol concentrations greater than and triglyceride concentrations less than serum values.
Amylase and Lipase: Peritoneal fluid amylase and lipase activity are less than paired serum values in the healthy horse.34 Inversion of this relationship may occur with pancreatitis, cholelithiasis, small intestinal mucosal injury, or rupture of the small intestine.34
Phosphate: Peritoneal fluid phosphate concentrations have been suggested to be useful in detection of major intestinal injury.40,41 Horses requiring surgical resection of necrotic bowel associated with severe colic had significantly greater peritoneal fluid phosphate concentrations than either normal horses or horses with medical causes of colic.40 Peritoneal fluid phosphate levels >3.6 mg/dl predicted the need for surgical intervention with a sensitivity of 77% and a specificity of 76%.40 This same report suggested that serum phosphate levels alone may be of value when peritoneal fluid specimens are unavailable. It is critical to use age-appropriate reference ranges, since foals may have greater serum and peritoneal fluid phosphate values, attributable to increased osseous metabolism.
ALP and AST: Activities of ALP and AST in equine peritoneal fluid are significantly less than that of serum activities in health. Because of the high tissue activity of these enzymes in liver and intestinal mucosa, elevated peritoneal fluid values have been suggested to potentially reflect damage to these organs. Lack of tissue specificity limits diagnostic reliability, however, and studies have shown that much of the ALP activity in adult equine peritoneal fluid is granulocytic in origin.20
Another potential application of fluid ALP activity is in the diagnosis of equine dysautonomia (grass sickness).2,21 This condition may mimic surgical causes of colic both in clinical signs and in peritoneal fluid abnormalities. In a study comparing fluid ALP activities in normal horses with those from horses with grass sickness and either medical or surgical causes of colic, significantly greater ALP activity was detected in peritoneal fluid from surgical cases of colic.21 Peritoneal fluid ALP activity from grass sickness cases was greater than that of normal horses but much less than that of surgical colic cases, aiding in differentiating these disorders. Peritoneal fluid values for ALP and AST in clinically normal foals and adult horses are reported in Table 9-2.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree