CHAPTER 8 Pleural Fluid
Thoracentesis is diagnostically indicated when there is physical, radiographic, or ultrasonographic evidence of pleural effusion or pleural neoplasia. Within the United States, pleuropneumonia is the most common cause of equine pleural effusion (termed parapneumonic effusions).1,2 For this reason, thoracentesis may be a useful part of the clinical evaluation of horses with lower respiratory tract disease.3,4 Occasionally, other underlying systemic disease results in development of pleural effusion; such effusions are termed nonparapneumonic effusions. Rarely, no underlying etiology is identified (called idiopathic pleural effusions). Both nonparapneumonic and idiopathic effusions are generally associated with a poorer prognosis.1,5
Clinical signs associated with pleural effusions vary, depending on the severity and duration, and may be fairly nonspecific. These include anorexia, depression, weight loss, exercise intolerance, pneumonia, dyspnea, dependent edema of the brisket and forelegs, and colic. Signs of colic may be attributable to actual concurrent gastrointestinal disorders or to severe pleurodynia (pleural pain) mimicking colic.6 Pleurodynia signs may also mimic exertional rhabdomyolysis or laminitis. There may be a history of recent stress such as travel, training, or hospitalization. Thoracentesis may be a worthwhile procedure when findings from physical examination (including rectal palpation), hematologic examination, serum biochemistry assays, fecal examination, and abdominocentesis have not indicated a diagnosis.
The dynamics of pleural fluid production in horses are thought to be different from those in dogs, cats, and people.7 In dogs and cats, the parietal pleura is supplied by systemic arteries, while the visceral pleura is supplied by pulmonary arteries, which are at a lower pressure. As a result, there is a net flow of pleural fluid from the parietal to visceral pleura. In horses, both pleurae are supplied by the systemic circulation. For the parietal surface this is mainly from intercostal arteries, while for the visceral surface, it is from bronchial, esophageal, and internal thoracic arteries.7,8 Hence, there is no net flow of pleural fluid from one surface to the other in horses, and pleural fluid is removed by the numerous lymphatic vessels of the pleurae. Most of the parietal surface is drained through the sternal lymph nodes, while the mediastinal and diaphragmatic portions are drained through the mediastinal lymph nodes. The visceral pleura is drained through the tracheobronchial lymph nodes.7,8
An increase in fluid volume results when the rate of fluid formation exceeds that of fluid removal. Pleural effusions have been reported in horses with a variety of conditions, including heart failure, chronic hepatic disease, hypoalbuminemia, diaphragmatic rupture, pleuritis, pneumonia, pulmonary abscesses, thoracic neoplasia, traumatic injury, parasitism, and hemothorax3,8–11 (Table 8-1).
TABLE 8-1 Differential Diagnosis for Pleural Effusion in the Horse*

* From Chaffin: Thoracentesis and pleural drainage in horses. Equine Vet Ed 10(2):106-108, 1998.
Specimen Collection
Thoracentesis may be performed in the standing horse with minimal restraint. Specific collection techniques have been detailed elsewhere.2,12,13 A bridle, halter, and stocks are usually adequate. If necessary, additional manual restraint and/or tranquilization may be used. Aseptic technique is employed. Most horses have a fenestrated mediastinum, and so fluid from either side is anticipated to be similar in the healthy horse. In disease conditions of the thorax, mediastinal fenestrations may become obstructed with fibrin; consequently, the two sides may not be affected to the same degree. It is therefore advisable to tap both sides, beginning with the more severely affected hemithorax. On the right side this is usually done at the sixth or seventh intercostal spaces, up to 10 cm dorsal to the level of the olecranon or the costochondral junction.3,7,11,14 On the left side this is usually done at the sixth to ninth intercostal spaces, 4 to 6 cm dorsal to the level of the olecranon or just dorsal to the costochondral junction.7,14 Entry just cranial to the rib margin prevents laceration of the intercostal vessels and nerves that course along the caudal aspect of the rib.13 Care should be taken to avoid the lateral thoracic vein.6,15 Thoracic auscultation, percussion, radiography, and/or ultrasonography may be useful to determine the location(s) of an effusion in many cases.7,14,16,17 This may influence the site selected for thoracentesis.
Pleural fluid can usually be readily obtained on the first attempt when an effusion is present. However, if no fluid is collected, another site should be selected. If this is also unsuccessful, a 30-cm bitch catheter can be used.11 Such difficulty in collecting a sample probably means the volume of fluid present is not increased. However, it could also mean that the effusion is loculated rather than diffuse. Radiography and (particularly) ultrasonography are useful ancillary techniques to visualize such loculation and to guide thoracentesis.17
Using standard techniques, 2 to 8 ml of pleural fluid can be obtained from most normal horses.11 Only 1 or 2 ml of fluid is usually required for a total nucleated cell count, cytologic examination, total protein measurement, and bacteriologic testing (if necessary).
Complications of Thoracentesis
Complications of thoracentesis are considered uncommon,14 and no undesirable sequelae were observed in one study of 18 clinically normal horses.11 Improper technique may result in pneumothorax, lung laceration, hemothorax, cardiac arrhythmia, or puncture of bowel, liver, or heart.6,13 Mild pneumothorax resulting from aspiration of air through the cannula is commonly asymptomatic, and any free air within the thoracic cavity is rapidly reabsorbed.5 Occasionally, localized cellulitis surrounding the thoracentesis site requires symptomatic treatment.15
When draining a large volume of effusion, intravenous fluid support should be provided to prevent hemoconcentration. Unless severe respiratory distress warrants rapid drainage, fluid removal should be slow to prevent development of pulmonary edema.5
Specimen Handling Considerations
Fluid should be collected into an EDTA tube for cell counts, cytologic examination, and (refractometric) total protein measurement. With small sample size (EDTA tubes less than one quarter filled), erroneous results for fluid protein levels and cell counts may be obtained (see section on biochemical examination).18 Aside from protein analysis, other biochemistry tests are only occasionally performed on pleural fluid in select diagnostic situations.
Gross Fluid Examination
Gross visual inspection of fluid consists of noting fluid volume, color, turbidity, odor, and clot formation. Normal, nonhemodiluted pleural fluid will not clot, is of small volume, and is clear to slightly hazy, pale straw yellow, and odorless.11,13
Subjective assessment of the color, turbidity, odor, and volume (as assessed by the ease or rate of collection) of pleural fluid in the thoracic cavity may often provide a provisional diagnosis and thus allow initiation of therapy before complete laboratory results are obtained. If the pleural fluid is grossly normal in appearance, it is probably normal, since most pleural effusions of the equine are exudative and will be visibly abnormal.1 However, when samples are moderately contaminated by peripheral blood at collection, such diagnostic inferences are more difficult. Evaluating the gross appearance of pleural fluid should not be a substitute for total nucleated cell counts, cytologic examination, and total protein determination. However, in many cases, particularly those without significant hemorrhage, such assessments allow the fluid to be broadly categorized as originating from a transudative or exudative process.
Volume
It is usually possible to obtain a few milliliters of pleural fluid from a healthy horse, though this may not be possible at the first attempt. Some normal horses may have no retrievable fluid.13 Of 18 clinically normal horses, 17 yielded 2 to 8 ml of pleural fluid on thoracentesis.11
Odor, Color, and Turbidity
Normal pleural fluid is odorless and sterile. A foul odor may accompany tissue necrosis or anaerobic bacterial infection.13 However, absence of foul odor does not rule out either of these possibilities.
Normal pleural fluid and transudative effusions are clear and colorless to pale yellow because of their low cellularity. Exudative effusions are more likely to be discolored and turbid, attributable to their increased cellularity and protein content. In these circumstances, it is diagnostically useful to grossly examine the sample’s sediment and supernatant. In the field situation, this can be done by allowing the fluid to sediment by gravity. In the laboratory, a microhematocrit centrifuge or a regular centrifuge can be used. The height of the sediment in the tube is usually proportional to the cellularity of the fluid, while its color varies with the relative numbers of RBCs and nucleated cells present. It may be red when RBCs predominate or brown to gray to off-white when most cells are WBCs. Red (hemolytic), port wine, amber, red-brown, or brown discoloration of the supernatant usually reflects damage to RBCs and sometimes WBCs that occurred before collection.
Shades of pink to red discoloration will occur with the presence of red cells or free hemoglobin in the specimen. Changes in red discoloration during specimen collection can alert the veterinarian to possible peripheral blood contamination. Uniformly red discolored specimens generally represent true hemorrhagic effusions and may be associated with bleeding diatheses, major vessel laceration, tumor or abscess rupture, traumatic injury, pulmonary infarction, or lung lobe torsion.13,19 Pneumothorax may accompany some causes of hemothorax. When the specimen grossly resembles whole blood, determination of packed cell volume (fluid and venous) and clotting times, as well as cytologic appearance, may be useful. Failure of the specimen to clot or presence of significant erythrophagia upon microscopic examination suggests true hemothorax. Contaminated specimens most often have a packed cell volume significantly less than that of peripheral blood, and platelet clumps may be visualized microscopically.
Reddish brown, port wine, or muddy effusions may be associated with ischemic tissue injury/necrosis or neoplasia.13 Presence of degenerative leukocyte changes (loss of nuclear segmentation, indistinct nuclear margins) with concurrent presence of bacterial organisms is compatible with sepsis.
Cytologic Examination
A cell count, total protein determination, and cytologic evaluation can be performed in the laboratory. Such tests require little equipment and yield much useful information. The method by which smears are prepared in the laboratory for cytologic evaluation varies with the specimen’s cellularity and the equipment available. Fluids with a total nucleated cell count less than 5000/μl are most readily examined if smears are made after cells are concentrated by some means. In diagnostic laboratories, special cytocentrifuges are often used for this purpose. A 100-μl aliquot yields ideal cell morphology for cytospin examination of most fluid specimens. (See Figs. 9-1 and 9-2.) Transudative, very-low-cellularity effusions may require up to 200 μl to improve cellularity for microscopic examination. Extremely high-cellularity, exudative effusions may require a reduced volume aliquot (25 to 50 μl) or specimen dilution to achieve cytospin preparations with ideal cell morphology and numbers.
In the practice laboratory without a cytocentrifuge, specimen concentration is achieved by centrifugation of up to 10 ml of pleural fluid in a tube for about 5 minutes at 1000 to 1500 rpm. The resultant supernatant is removed and saved for total protein measurement. The sedimented cells are then gently resuspended in about 0.25 to 0.5 μl of pleural fluid and smears prepared, often using a line smear technique to concentrate cells at the leading edge of the smear (see Chapter 1). Romanowsky-type stains are usually used, such as Wright’s, May-Grünwald, Giemsa, or Diff-Quik. Fluids with a total nucleated cell count of 5000 to 10,000/μl may be prepared from centrifuged or uncentrifuged specimens, depending on the examiner’s preference. (Cytospin preparations or line smears from centrifuged sediments yield more cellular and therefore more easily scanned smears in these situations.) When the total nucleated cell count is >10,000/μl and the fluid’s turbidity is therefore greater than normal, direct smears of uncentrifuged specimens are usually satisfactory.
Cell Counts and Cytologic Examination
Total nucleated cell and erythrocyte counts of pleural fluid are performed as for a blood sample. Depending on laboratory resources, this can vary from manual dilution with microscopic enumeration to the use of automated cell counters. Reference values are detailed in Table 8-2. Small-volume specimens (EDTA tube less than one quarter filled) may be sufficiently diluted to mildly decrease cell counts.18
TABLE 8-2 Reference Values for Equine Pleural Fluid*
| Measurement | Observed range | Comments |
|---|---|---|
| RBC count | 22,000–540,000/μl 22–540 × 109 L | ≤ 370,000/μl (≤ 370 × 109 L) in 94% of horses |
| Total nucleated cell count | 800–12,100/μl 0.8–12.1 × 109 L | ≤ 8000/μl (≤ 8.0 × 109 L) in 94% of horses |
| Differential cell count | ||
| Neutrophils | 450–10,290/μl 0.5–10.3 × 109 L 32%–91% | 450–7120/μl (≤ 0.5–7.1 × 109 L) in 94% of horses |
| Lymphocytes | 0–680/μl 0–0.7 × 109 L 0%–22% | 0%–10% in 94% of horses |
| Large mononuclear cells | 50–2620/μl 0.1–2.6 × 109 L 5%–66% | |
| Eosinophils | 0–170/μl 0–0.2 × 109 L 0%–9% | No eosinophils observed in 89% of horses; 0%–1% in 94% of horses |
| Specific gravity | 1.008–1.031 | |
| Total protein | 0.2–4.7 g/dl 2–47 g/L | ≤ 2.5 g/dl (25 g/L) in 83% of horses; ≤ 3.4 g/dl (≤ 34 g/L) in 94% of horses |
* Values derived from 18 clinically normal horses. From Wagner and Bennett: Analysis of equine thoracic fluid. Vet Clin Pathol 11(1):13-17, 1982.
Though RBCs are present in pleural fluid collected from clinically normal horses, they are considered to be the result of contamination by minor hemorrhage from intercostal muscles. Accordingly, erythrophagocytosis is not a feature of normal pleural fluid.11 The fluid supernatant is also clear and nonhemolyzed in normal specimens. Erythrocyte counts are not often performed on pleural fluid unless automated techniques are used that routinely include red cell number determination.
Nucleated cells are generally categorized as neutrophils, lymphocytes, large mononuclear cells (including monocytes, macrophages, and mesothelial cells), eosinophils, basophils, or mast cells. Differential counts are usually performed on 100 to 200 cells. Though the numbers of each cell type are usually expressed as a percentage value, these figures must be related to the total nucleated cell count, total protein concentration, and volume of fluid present for accurate interpretation. Assessment of cell morphology is a very important part of cytologic examination. General comments pertaining to peritoneal fluid leukocyte morphology are probably applicable to pleural fluid, though there is a paucity of such information readily available.
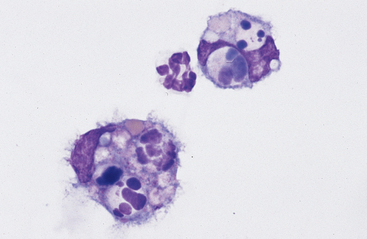
Neutrophils: Neutrophils that enter the thoracic cavity (as with neutrophils entering other body cavities or tissues) do not return to the bloodstream. Consequently, cell aging and death are normal events. Aged neutrophils are often moderately hypersegmented to pyknotic,20–23 and leukophagocytosis of senescent neutrophils by macrophages may be infrequently observed (Fig. 8-1). This finding may be difficult to distinguish from occult or mild inflammation. Neutrophils in normal pleural fluid do not exhibit phagocytic activity per se.
The presence of band neutrophils or more immature granulocytic cells suggests acute inflammation and mobilization of the neutrophil storage and maturation pools. Presence of degenerative changes (such as cell swelling, loss of nuclear segmentation, and indistinct nuclear margins)24 suggests a harsh pleural environment. This may occur secondary to the presence of bacterial cytotoxins within the thoracic cavity. Toxic changes (such as increased cytoplasmic basophilia, vacuolation, or Dohle bodies) may also be observed in septicemia or enterotoxemia. Such toxic change is considered to be “preexisting,” occurring during myelopoiesis, rather than subsequent to migration into the pleural cavity. These changes, accompanied by visualization of phagocytized bacterial organisms (Figs. 8-2, 9-13, and 9-14) are compatible with septic pleuritis.
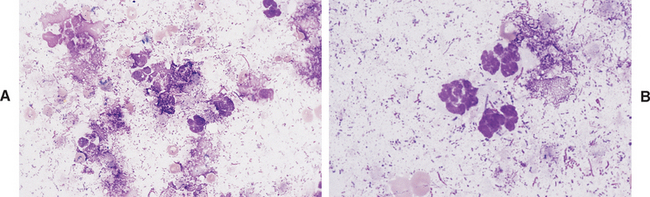
Fig. 8-2 Equine septic pleuritis.
A, Degenerate neutrophils, smudged cells, and low numbers of RBCs are present within a background of mixed bacterial organisms. B, Higher magnification view. (Wright-Giemsa stain)
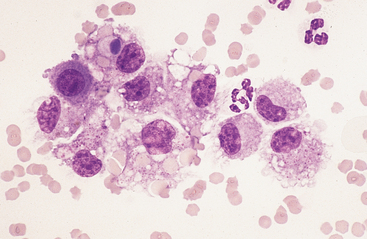
Large Mononuclear Cells: The category of large mononuclear cells incorporates nonreactive (tissue) macrophages of blood monocyte origin, reactive (tissue) macrophages, and mesothelial cells (see Figs. 9-2 and 9-4 to 9-6). As in peritoneal fluid, these cells are often difficult to distinguish morphologically.11 They are conveniently grouped together and often referred to collectively as mononuclear phagocytes, since all have phagocytic potential (Figs. 8-1 and 8-3). All of these cells are large, usually with a moderate to high nuclear to cytoplasmic ratio, and abundant, somewhat basophilic cytoplasm (see Figs. 9-2 and 9-4). The nucleus is their most distinctive feature, but even that is not particularly characteristic, and subclassification of large mononuclear cells is quite subjective.
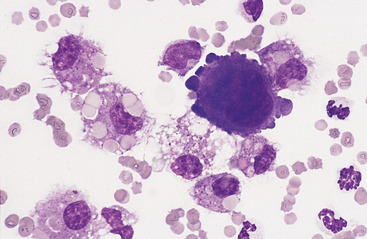
Mesothelial cells usually have an oval nucleus with a finely reticular chromatin pattern. When individualized, a fine eosinophilic “corona” or halo of glycocalyx may be evident along the cell margin (Fig. 8-5). In transudative effusions, they may occur in sheets or rafts, have a uniform appearance, and have a polygonal to rhomboid shape. In exudative effusions, mesothelial cells may become reactive or transformed and exhibit features suggesting increased proliferation, including increased cytoplasmic basophilia, multinucleation, prominent nucleoli, and mitotic activity (Figs. 8-4 and 8-5). Hyperplastic/dysplastic features can begin to mimic neoplasia in severe inflammatory conditions. Large numbers of mesothelial cells should raise suspicions of mesothelioma.13
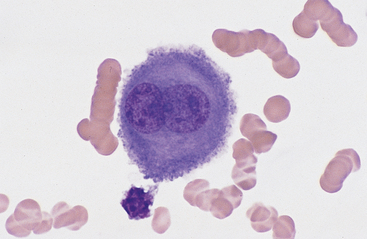
Fig. 8-5 Equine pleural fluid.
A single mesothelial cell is present. Note the increased cytoplasmic basophilia, binucleation, and ruffled cytoplasmic border, all of which support reactive transformation. (Wright-Giemsa stain)
Reactive cells tend to have more abundant, more basophilic cytoplasm. Reactive macrophages often have ruffled cytoplasmic margins, prominent cytoplasmic vacuoles, and/or inclusions (phagosomes). The latter may be unidentifiable debris or degenerating inflammatory cells or RBCs (see Figs. 8-1 and 8-3). Reactive large mononuclear cells were not observed in pleural fluid samples of 18 clinically normal horses.11 In acute inflammatory effusions, the relative percentage of monocytes/macrophages decreases with increasing granulocytic cell numbers. In more chronic effusions, mononuclear/macrophages are typically present in increased percentages and may exhibit reactive changes.
An increased lymphocyte percentage may occur in chronic inflammatory conditions, chylothorax (particularly acutely), or neoplasia. Lymphoblasts are not observed in normal pleural fluid. These cells have a densely staining, coarsely clumped chromatin pattern, possibly with obvious nucleoli, and intensely basophilic cytoplasm that may contain small to large vacuoles. Cytologic diagnosis of lymphoma is usually based on the presence of large numbers of such cells.13 Plasma cells are not a normal finding and may reflect chronic antigenic stimulation.
Neoplastic cells may be identified on pleural fluid cytology, although the relative diagnostic yield of this procedure for all tumor types may be low.1,25 Neoplastic round cells (most commonly lymphoblasts), mesothelial cells (mesotheliomas), and epithelial cells (carcinomas and adenocarcinomas) are those most commonly encountered25 (see Figs. 8-8 to 8-17). Criteria of malignancy include anisocytosis, anisokaryosis, variation in nucleus to cytoplasm ratio, increased cell size, nuclear gigantism, multinucleation, prominent/large/angular/multiple nucleoli, and increased/abnormal mitotic activity. Presence of concurrent inflammation may cloud distinction from reactive hyperplasia/dysplasia in some instances, necessitating further diagnostic investigation. Absence of identifiable neoplastic cells on pleural fluid cytology does not rule out neoplasia, since tumor cells do not consistently exfoliate into pleural effusions. In one study of 38 horses with thoracic neoplasia, pleural fluid cytologic diagnosis was achieved in only 12 (32%), 10 of which had lymphoma.25 In another study evaluating only horses with lymphoma, 12 of 13 horses examined had pleural effusion, and fluid cytology was diagnostic in 10.26 This suggests that a higher diagnostic yield for pleural fluid cytology is possible in cases of lymphoma than for other types of thoracic neoplasia. Given the fact that lymphoma accounts for over half of thoracic neoplasias in the equine25,26 and that concurrent pleural effusion is common,26,27 thoracentesis and pleural fluid cytology remain a valuable diagnostic tool.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree