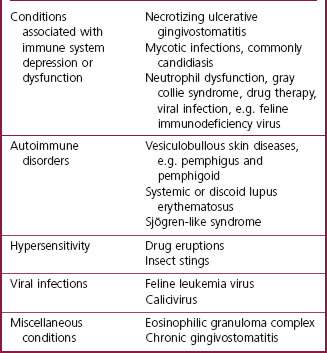
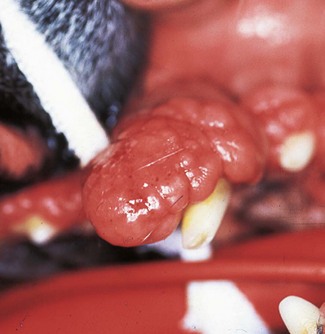
Chapter 9 Periodontal disease is the result of the inflammatory response to dental plaque, i.e. oral bacteria, and is limited to the periodontium. It is the most common oral disease seen in dogs (Hamp et al. 1984). It is also common in cats (Reichart et al. 1984). In fact, periodontal disease is probably the most common disease seen in small animal practice with the great majority of dogs and cats over the age of 3 years having a degree of disease that warrants intervention. In addition to periodontal disease, a spectrum of inflammatory responses to agents other than plaque (e.g. toxic, viral and unknown) also occurs in the oral cavity. These generally affect the oral mucous membrane, but may also involve the periodontium. Inflammation of the oral mucosa is called stomatitis. Table 9.1 lists the most important oral inflammatory conditions, other than periodontal disease. This chapter will deal with periodontal disease and feline gingivostomatitis. Periodontal disease is a collective term for a number of plaque-induced inflammatory lesions that affect the periodontium. The term infection refers to the presence and multiplication of a microorganism in body tissues. Periodontal disease is a unique infection in that it is not associated with a massive bacterial invasion of the tissues. Gingivitis is inflammation of the gingiva and is the earliest sign of disease. Individuals with untreated gingivitis may develop periodontitis. The inflammatory reactions in periodontitis result in destruction of the periodontal ligament and alveolar bone. The result of untreated periodontitis is ultimately exfoliation of the affected tooth. Thus, gingivitis is inflammation that is not associated with destruction (loss) of supporting tissue. It is reversible. In contrast, periodontitis is inflammation where the tooth has lost a variable degree of its support (attachment). It is irreversible. The salient features of gingivitis and periodontitis are depicted in diagrammatic form in Figure 9.1. Fig. 9.1 Periodontal disease Periodontal disease can cause discomfort to affected individuals. Moreover, there is strong circumstantial evidence that a focus of infection in the oral cavity may cause disease of distant organs (DeBowes et al. 1996). Consequently, prevention and treatment of periodontal disease is important for the general health of companion animals. It is not a cosmetic issue. Prevention of periodontal disease is detailed in Chapter 10. This chapter details etiology, pathogenesis, diagnosis and treatment. Successful management of periodontal disease relies on a comprehensive understanding of the etiology and pathogenesis of the disease. Dental plaque is a biofilm composed of aggregates of bacteria and their by-products, salivary components, oral debris and occasional epithelial and inflammatory cells (Fig. 9.2). Plaque accumulation starts within minutes on a clean tooth surface. The initial accumulation of plaque occurs supragingivally but will extend into the sulcus and populate the subgingival region if left undisturbed. As demonstrated in a study where dogs were fed by intubation, the formation of dental plaque occurs whether food passes through the oral cavity or not, i.e. food debris does not attach to the teeth to form plaque (Egelberg 1965). Supragingival plaque bacteria derive their main nutrients from dietary particles dissolved in saliva. Within the sulcus or pathologic periodontal pocket, the major nutritional source for bacterial metabolism comes from the periodontal tissues and blood. Fig. 9.2 Dental plaque Classic experiments have demonstrated that accumulation of plaque on the tooth surfaces reproducibly induces an inflammatory response in associated gingival tissues, and that removal of the plaque leads to disappearance of the clinical signs of this inflammation (Löe et al. 1965; Theilade et al. 1966). At first, a direct relationship was assumed to exist between the total number of bacteria that accumulated on a tooth surface and the amplitude of the pathogenic effect. Such a view of dental plaque as a biomass is referred to as the non-specific plaque hypothesis (Theilade 1986). As it became evident that not all gingivitis lesions invariably developed to periodontitis lesions, the specific plaque hypothesis was developed. In this hypothesis, the view is that periodontitis is caused by specific pathogens (Loesche 1979). Differences in the composition of the subgingival plaque have been attributed in part to the local availability of blood products, pocket depth, redox potential and PO2. Therefore, the question of whether the presence of specific microorganisms in patients or distinct sites may be the cause or consequence of disease is still a matter of dispute (Socransky et al. 1987). Many periodontopathogens are strict anaerobes and, as such, may contribute little to the initiation of periodontitis in shallow periodontal pockets. Instead, these organisms are linked to progression of disease in sites with pre-existing periodontitis. The plaque associated with healthy gingiva mainly comprises aerobic and facultative anaerobic bacteria. As gingivitis develops, plaque extends subgingivally. Aerobes consume oxygen and a low redox potential is created, which makes the environment more suitable for growth of anaerobic species. The aerobic population does not decrease, but with increasing number of anaerobes, the aerobic/anaerobic ratio decreases. The subgingival flora associated with periodontitis is predominantly anaerobic and consists of Porphyromonas spp, Prevotella spp, Peptostreptococcus spp, Fusobacterium spp and spirochetes (Hennet and Harvey 1991). High levels of Porphyromonas spp and spirochetes are consistently associated with progressive periodontitis in the dog. The bacterial flora of the normal feline gingival margin, as well as the bacteria found in subgingival plaque of cats with gingivitis and periodontitis, are similar to those found in humans and dogs under similar conditions (Mallonee et al. 1988; Love et al. 1990). Dental calculus is mineralized plaque. However, a layer of plaque always covers calculus. Both supragingival and subgingival plaque becomes mineralized. Supragingival calculus per se does not exert an irritant effect on the gingival tissues. In fact, it has been shown in monkeys that a normal attachment may be seen between the junctional epithelium and calculus if the calculus surface had been disinfected using chlorhexidine (Listgarten and Ellegaard 1973). It has also been shown that sterilized calculus may be encapsulated in connective tissue without causing marked inflammation or abscess formation (Allen and Kerr 1965). It has been speculated that calculus may exert a detrimental effect on the soft tissue owing to its rough surface. However, it has clearly been established that surface roughness alone does not initiate gingivitis (Waerhaug 1956). The main importance of calculus in periodontal disease thus seems to be its role as a plaque-retentive surface. This is supported by well-controlled animal (Nyman et al. 1986) and human (Nyman et al. 1988; Mombelli et al. 1995) studies that have shown that the removal of subgingival plaque on top of subgingival calculus will result in healing of periodontal lesions and the maintenance of healthy periodontal tissues. The pathogenic mechanisms involved in periodontal disease include: • Direct injury by plaque microorganisms • Indirect injury by plaque microorganisms via inflammation. The microbiota in periodontal pockets is in a continual state of flux; periodontitis is a dynamic infection caused by a combination of bacterial vectors that change over time. As a result, the molecular events that trigger and sustain the inflammatory reactions constantly change. Many microbial products have little or no direct toxic effect on the host. However, they possess the potential to activate non-immune and immune inflammatory reactions that cause the tissue damage. It is now well accepted that it is the host’s response to the plaque bacteria, rather than microbial virulence per se that directly causes the tissue damage (Kinane and Lindhe 1997). In gingivitis, the plaque-induced inflammation is limited to the soft tissue of the gingiva (Fig. 9.1A). Sulcus depths are normal (i.e. periodontal probing depths are 1–3 mm in the dog and 0.5–1.0 mm in the cat). As periodontitis occurs (Fig. 9.1C), the inflammatory destruction of the coronal part of the periodontal ligament allows apical migration of the epithelial attachment and the formation of a pathologic periodontal pocket (i.e. periodontal probing depths increase). If the inflammatory disease is permitted to progress, the crestal portion of the alveolar process begins to resorb. Alveolar bone destruction type and extent are diagnosed radiographically. The resorption may proceed apically on a horizontal level. Horizontal bone destruction is often accompanied by gingival recession, so periodontal pockets may not form (Fig. 9.1D). If there is no gingival recession, the periodontal pocket is supra-alveolar, i.e. above the level of the alveolar margin. The pattern of bone destruction may also proceed in a vertical direction along the root to form angular bony defects. The periodontal pocket is now intra- or subalveolar, i.e. below the level of the crestal bone. Other conditions, such as physical or psychologic stress and malnutrition, may impair protective responses such as the production of antioxidants and acute phase proteins, and can aggravate periodontitis but do not actually cause destructive tissue inflammation. A genetic predisposition to destructive inflammation of the periodontium may be important in some individuals. In humans, a strong association has been observed between the severity of periodontitis and a specific genotype of the interleukin-1 (IL-1) gene cluster (Kornman et al. 1997). Patients carrying this periodontitis-associated genotype may demonstrate phenotypic differences, as indicated by elevated levels of IL-1β in gingival sulcular (crevicular) fluid (Engebretson et al. 1999). No similar data are available for the dog or cat. Oral examination and recording of findings are detailed in Chapter 6. The following parameters need to be assessed and recorded for each tooth in all patients: Gingivitis manifests clinically as swelling, reddening and often bleeding of the gingival margin (Fig. 9.3). It may be accompanied by halitosis. It is diagnosed clinically by means of a combination of visual inspection and tactile examination. The presence and degree of gingival inflammation is assessed based on a combination of redness and swelling, as well as presence or absence of bleeding on gentle probing of the gingival sulcus. Various indices can be used to give a numerical value to the degree of gingival inflammation present. In the clinical situation, a simple bleeding index may be the most useful. Using this method the gingival sulcus of each tooth is gently probed at several points and given a score of 0 if there is no bleeding and a score of 1 if the probing elicits bleeding. The patient with uncomplicated gingivitis will have normal periodontal probing depths (1–3 mm in the dog and 0.5–1.0 mm in the cat) and show no evidence of gingival recession, furcation involvement or tooth mobility. Radiography is not mandatory if the clinical examination reveals no evidence of periodontal destruction, i.e. periodontitis. Fig. 9.3 Gingivitis Gingival hyperplasia (Figs 9.1B, 9.4) may be the result of plaque-induced inflammation, i.e. hyperplastic gingivitis. It may also be of idiopathic or familial origin, and it can be induced by certain drugs, e.g. hydantoin, ciclosporins. Gingival hyperplasia is common in some breeds, e.g. Boxer, Springer Spaniel. There is an increase in periodontal probing depths owing to the gingival overgrowth. Halitosis is common and is often the first sign noted by the pet owner. Large amounts of dental deposits are usually present. These deposits need to be removed to allow a detailed examination of the periodontium. Ulcers affecting mucous membranes of lips and cheeks may be present in areas where these tissues are exposed to plaque-covered tooth surfaces (Fig. 9.5). Fig. 9.5 Gingival recession and mucous membrane ulceration Tissue destruction in periodontitis is assessed by measuring periodontal probing depth, gingival recession, furcation involvement and degree of tooth mobility. In many cases, measuring or calculating the periodontal attachment level (PAL) is also useful. The PPD is not necessarily correlated with severity of attachment loss (Fig. 9.6). Gingival hyperplasia may contribute to a deep pocket (or pseudopocket if there is no attachment loss), while gingival recession may result in the absence of a pocket but also minimal remaining attachment. PAL records the distance from the cemento-enamel junction (or from a fixed point on the tooth) to the base or apical extension of the pathologic pocket. It is thus a more accurate assessment of tissue loss in periodontitis. PAL can either be measured with a periodontal probe or it can be calculated (e.g. PPD + gingival recession). Fig. 9.6 Attachment loss Radiography to assess the type and extent of alveolar bone destruction is mandatory for periodontitis patients. Consequently, full mouth radiographs should be performed prior to the institution of any therapy. In addition, radiographs need to be taken at regular intervals to monitor outcome of any treatment. A detailed examination of the periodontal ligament space and interproximal alveolar margin requires the use of an intraoral radiographic technique (detailed in Ch. 7). The radiographic changes associated with periodontal disease include resorption of the alveolar margin, widening of the periodontal space, a break in the path or loss of the radiopacity of the lamina dura and destruction of alveolar bone resulting in supra- or infrabony pockets. Radiographs using a parallel technique (see Ch. 7) will demonstrate more accurately the features of periodontitis because this technique provides a better view of the alveolar margin and reveals more accurately the actual extent or depth of the periodontal lesion in relation to the root of the tooth. Radiographs produced with a bisecting angle technique may show greater destruction of the alveolar bone than is actually present, because the central ray is directed obliquely to the long axis of the teeth and jaw, which produces dimensional distortion. Moreover, with the bisecting angle technique, subgingival calculus may be superimposed on alveolar bone and would thus not be detected. Views taken using a parallel technique will demonstrate deposits of subgingival calculus and defects of the cementum but may not cover a sufficient area to demonstrate extensive periodontitis lesions adequately. In the maxilla and anterior mandible, bisecting angle and parallel views of the same region may be required to visualize the extent of the tissue destruction more accurately. As periodontitis develops, the crestal portion of the alveolar process begins to resorb. Radiographically, the destruction is evident as a cup-shaped notch or as scalloping of the alveolar margin. The resorption may proceed apically on a horizontal level (Fig. 9.7). Beyond this, the lamina dura appears to be normal and there is no widening of the periodontal space. Horizontal bone destruction (Fig. 9.1D) is often accompanied by gingival recession (Fig. 9.5), so periodontal pockets may not form. If there is no gingival recession, the periodontal pocket is supra-alveolar, i.e. above the level of the alveolar margin. The pattern of bone destruction may also proceed in a vertical direction along the root to form angular bony defects. Radiographically these are usually evidenced by a vertical or V-shaped flaw, with the root of the tooth forming one side of the defect (Fig. 9.8). The periodontal pocket is now infra- or subalveolar, i.e. below the level of the crestal bone (Fig. 9.1C). Fig. 9.7 Horizontal bone loss
Periodontal disease
Introduction
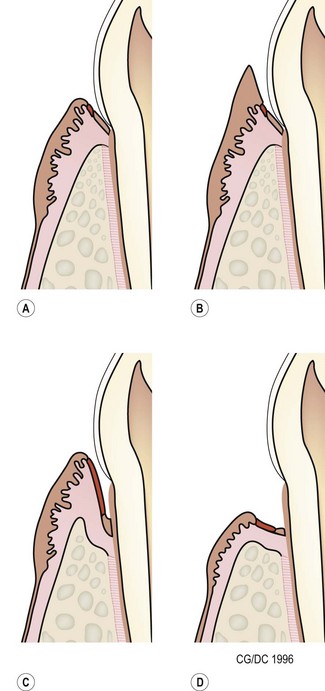
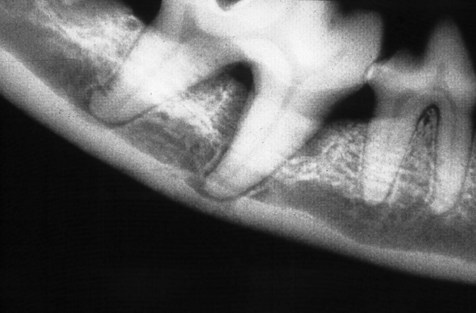
Periodontal disease is a collective term for plaque-induced inflammation of the gingiva. (A) Gingivitis. The inflammation is limited to the gingiva with no associated destruction of the periodontium. Gingivitis is reversible. (B) Gingival hyperplasia. Gingival hyperplasia may be the result of plaque-induced inflammation (hyperplastic gingivitis), but may also be of idiopathic or familial origin. It can also be induced by certain drugs. Gingival hyperplasia results in increased periodontal probing depths, initially with no loss of periodontal support, i.e. there is no attachment loss. (C) Periodontitis with vertical bone loss. The plaque-induced inflammation results in irreversible destruction of the periodontal ligament and alveolar bone. The junctional epithelium (epithelial attachment) migrates apically and attaches on the root surfaces. If the gingival margin does not recede, the apical migration of the epithelial attachment results in increased periodontal probing depth, i.e. a pathologic pocket is formed. Destruction of the alveolar bone can be horizontal or vertical. Shown here is vertical bone loss, resulting in the formation of a periodontal pocket where the apical extension of the pocket is below the margin of the alveolar bone, i.e. infrabony pocket. (D) Periodontitis with horizontal bone loss. The periodontal destruction is evidenced by loss of periodontal ligament and horizontal bone loss. The junctional epithelium has migrated apically and attached to the root surfaces. However, the gingival margin has receded, so periodontal probing depths do not increase.
Etiology
Dental plaque
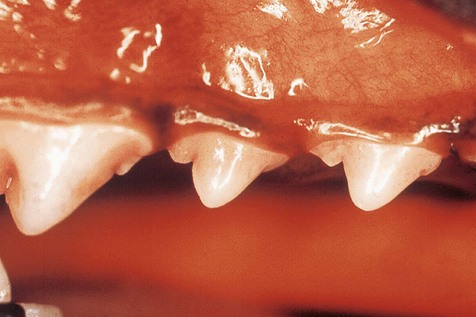
Dental plaque is a biofilm composed of aggregates of bacteria and their by-products, salivary components, oral debris and occasional epithelial and inflammatory cells. It starts accumulating within minutes on a clean tooth surface Plaque may be difficult to see with the naked eye and the use of plaque-disclosing solutions (dyes that stain plaque) is recommended for visualization.
Dental calculus
Pathogenesis
Diagnosis
Gingivitis
Clinical signs and diagnostic methods
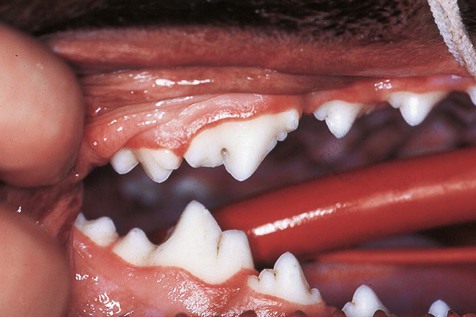
Gingivitis manifests clinically as swelling and reddening of the gingival margin.
Periodontitis
Clinical signs
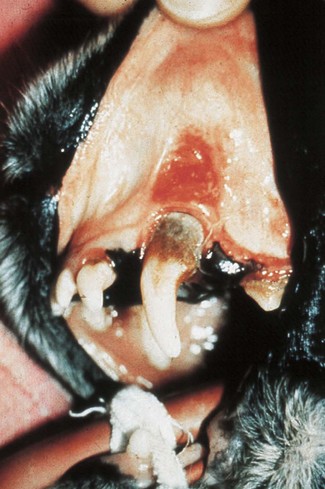
The periodontal ligament and alveolar bone on the labial aspect of the left upper canine has been destroyed. The gingival margin has receded. Periodontal probing depth is 1 mm, i.e. there is no pathologic pocket. A mucous membrane ulcer has developed on the lip surface that is in contact with the plaque-covered tooth surface. Note: While uncomplicated periodontitis is not associated with severe discomfort, these mucous membrane ulcers are known to be painful.
Diagnostic methods
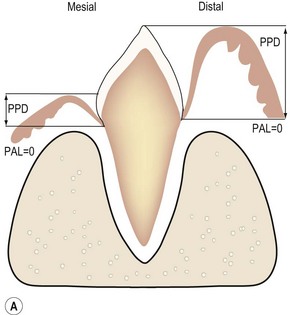
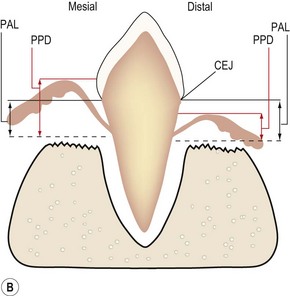
(A) The epithelial attachment on both sides of the tooth is at the cemento-enamel junction (CEJ), so there is no loss of periodontal attachment (PAL = 0). The surface labeled mesial depicts normal gingival attachment; periodontal probing depth (PPD) is 1–2 mm. The surface labeled distal has an increased PPD, e.g. 8 mm. However, this is not periodontitis as there has been no loss of periodontal support. (B) PPD on the surface labeled mesial is increased, e.g. 6 mm. PPD on the side labeled distal is normal, i.e. 1–2 mm, due to the gingival recession. PAL, i.e. the extent of periodontal ligament and alveolar bone destruction, is the same.
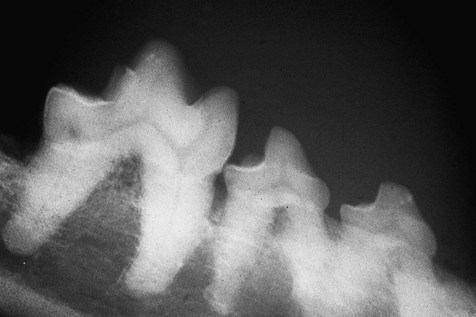
In this dog, resorption of the alveolar bone has proceeded apically in a horizontal fashion. The right mandibular 3rd premolar is unaffected, i.e. the height of the alveolar margin is normal. The right mandibular 4th premolar and 1st molar have lost around 2 mm of alveolar bone.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Periodontal disease