Sherrill A. Fleming, Consulting Editor The traditional approach to parasite control programs has focused on using the appropriate anthelmintic at appropriate intervals. Parasitic disease in domestic animals is assumed to be the result of not dosing the animals often enough with anthelmintics. Scant consideration has been paid to the interaction of the parasite with the host and the environment because of the reliance on anthelmintics. These drugs have been placed directly into the hands of the livestock owner because the expertise of a veterinarian did not seem necessary in the control of parasites. However, reports of resistance to anthelmintics and emergence of new manifestations of parasitism are surfacing throughout the world. It has become increasingly apparent over the past 20 years that this approach to parasite control is no longer sustainable. Secondary to the notion that parasitism is under control is the decrease in research to develop new anthelmintics. Currently there is little in pharmacologic development other than variations on the current anthelmintics. Research programs in parasitology of domestic animals are facing funding reductions as research priorities are shifted to other diseases. As producers and owners struggle to deal with the realities of anthelmintic resistance, veterinary medicine must reassess traditional approaches to parasite control programs. Veterinarians will need to reeducate themselves away from the traditional tools of deworming, anthelmintic rotation, and pasture rotation. Integrated management strategies incorporating selective use of anthelmintic agents, enhancement of host immunity to parasitic infection, and grazing/environmental management have become increasingly important in the design of sustainable parasite control programs. The impact of parasitic infection varies widely with geographic area and management system. General guidelines may be suggested for parasitic control, but it is inadvisable to adhere to any rigid anthelmintic schedules or even management recommendations. The best parasite control programs are those designed with the goals of the producer in mind, as well as the costs and returns of treatment. Other factors that must be considered include the animal’s environment, climatic variations, and geographic location. Although many producers and owners would like a “cookbook” approach to parasite control, these are rarely effective across the various management conditions. It is unfortunate that an epidemiologically and economically sound parasite control program designed for animals in one geographic area may be neither efficient nor effective in another location. The most important concept in the design of sound parasite control programs is the interaction of the parasite with the host and the environment. An understanding of the life cycle and epidemiology will suggest the most effective methods for parasite control. In this chapter parasite factors, host factors, and environmental factors affecting transmission and disease expression are discussed for each major class of parasites in each host species (horses, cattle, small ruminants). The methods of monitoring parasite infections and anthelmintic resistance are presented in detail. The classes of anthelmintics and their modes of action are discussed and, finally, coccidiosis in cattle and small ruminants is summarized at the end of the chapter. Martin Krarup Nielsen The changed approaches being promoted for control of equine gastrointestinal helminths are based on a number of scientific concepts outlined in the following section. The recommendations given today are based on experts’ best knowledge of parasite and host biology, as well as mechanisms of anthelmintic resistance. As time passes, some of these recommendations are likely to change as new information is generated. In essence, the traditional strategies used for equine parasite control were based on the best scientific evidence available at the time, and the recommendations now given are merely a response to the experiences gathered over the past decades. The concepts underlying the modern approaches for equine parasite control are presented in the following. Anthelmintic resistance can be defined as “the loss of treatment efficacy of a given anthelmintic formulation that used to have efficacy against the same parasite species and stage in the same host animal, at the same dosage, and by the same route of administration.” This sentence implies several things: (1) If a drug formulation never had efficacy against the parasite and stage in question, then veterinarians are not facing resistance. (2) In case of nonlabeled usage of anthelmintics, veterinarians cannot make any conclusions regarding resistance because they have no information about the expected level of efficacy. (3) Horses are always infected with several different species of parasites at the same time, and resistance often occurs in some, but not all, of these species. (4) The expected level of efficacy needs to be defined for each parasite species for each drug in order evaluate the possible presence of resistant parasites. The current levels of anthelmintic resistance in equine parasites are presented in Table 49-1. Generally speaking, resistance has been widely documented in cyathostomins (small strongyles) and Parascaris equorum. It is worth noticing that as a general pattern, the resistance profiles are almost completely complementary between the two parasite categories. The drugs that seem to still work well against P. equorum often have no or little efficacy against cyathostomins and vice versa. However, levels of anthelmintic resistance are constantly developing and this pattern may therefore be shifting. Recent work has documented signs of emerging ivermectin and moxidectin resistance in cyathostomins,1–4 and total drug failure is a realistic possibility in this parasite group. No new anthelmintic formulations with new modes of action are expected to be introduced to the equine market in the foreseeable future. The only available method for evaluating anthelmintic efficacy in horses is the fecal egg count reduction test (FECRT). This method is outlined in Box 49-1 along with some suggested cutoff values for determining anthelmintic resistance. Egg reappearance periods (ERPs) are defined as the period of time from anthelmintic treatment until parasite eggs are found in the feces again. Although ERPs could potentially be established for a number of different parasite categories, they are most often referred to for strongyle (i.e., cyathostomin) parasites. The ERPs were initially defined for each anthelmintic class to help identify the optimum interval between anthelmintic treatments, and this was the basis for launching the so-called interval dose program in the 1960s.5 Nowadays, ERPs are used for an entirely different purpose. It has been observed that the first sign of developing anthelmintic resistance to a given drug is often a gradual shortening of the ERP until reduced efficacies can finally be found with the FECRT. The most prominent example of this is the observed ERPs of ivermectin and moxidectin against cyathostomin parasites. Initially, ERPs were reported to be at least 8 and 12 to 16 weeks for ivermectin and moxidectin, respectively,6–8 but recent publications reported egg reappearance at around weeks 4 to 5 for both drugs.1, 4 Two necropsy-based studies have suggested that these observations were due to survival of luminal yet premature stages of cyathostomin larvae.2,3 Prepatent periods (PPPs) should not be confused with the egg reappearance periods defined earlier. While the latter defines a response to anthelmintic treatment, the PPPs are defined in the absence of anthelmintic intervention. The PPP for any given parasite is defined as the time elapsed from the uptake of the infective stage of the parasite until it reaches patency and starts shedding eggs to be detected in the feces. Thus the PPP represents the duration of the parasitic part of the life cycle. Table 49-2 presents PPPs for some of the most important equine helminth parasites. It should be noted that although the PPPs for cyathostomin parasites are generally relatively short, they can undergo arrested development for up to several years, which thereby dramatically lengthens the PPP. 9 TABLE 49-2 Prepatent Periods of Some Important Equine Parasites * Encysted larval stages can remain dormant for years. Information about PPPs can be taken into account for identifying optimal times for routine collection of fecal samples and anthelmintic intervention. Regardless of parasite and host species, distributions of parasites among their hosts always seem to follow the same pattern. Hosts of similar age and breed and kept under identical management grazing the same pasture can have widely different parasite burdens. Parasites never follow a symmetric normal distribution but are rather said to be overdispersed.10 Technically speaking, this means that the variance is larger than the mean, but in practical terms this has been translated to the so-called 20/80 rule.11 This refers to the observation that about 20% of the host animals harbor approximately 80% of the parasites. Similarly, about 20% of horses are shedding 80% of the total strongyle egg output. This phenomenon is most pronounced in adult horses, where a large majority is shedding low numbers of strongyle eggs and very few individuals can be observed with very high counts. This pattern has been found to be consistent in individual horses over time, and the tendency is particularly strong among horses with 0 or low strongyle egg counts.12–14 Taken together, these observations have become the foundation for the selective therapy principle covered later in this chapter. The term “parasite refugia” has become generally accepted as perhaps the most important factor affecting the rate of development of anthelmintic resistance in large animal parasites.15 Parasites in refugia are the parts of any given parasite population (including all stages, both internal and external) that are not exposed to the anthelmintic drug at the time of treatment. It can be said that these parasite stages in a way “escaped” the drug and can be viewed as refugees, hence the terminology. Logically, all stages on pasture, such as eggs and preinfective larvae, are always in refugia. In addition, animals left untreated can be viewed as contributing to the refugia, and some parasitologists therefore distinguish between “pasture refugia” and “animal refugia.” Finally, some anthelmintic formulations have no efficacy against some parasite stages within the horse, and these can be regarded as part of the refugia as well. Examples of this include pyrantel formulations, which have no efficacy against parasite stages present outside the gastrointestinal lumen, and ivermectin, which has no documented efficacy against encysted cyathostomin larvae. The parasites in refugia are hypothesized to play a role in diluting out resistant parasites whenever the animals are dewormed. A simplistic explanation of this concept is the larger the refugia, the more the dilution of resistant parasites, and the slower the development of resistance. In a theoretic example, consider a horse herd that is dewormed with a drug that has efficacy against all parasitic stages of the parasites, and the treatments are carried out at a time of the year when no eggs or larvae are present on pasture. This would represent a scenario with no parasite refugia. Because no anthelmintic treatment is 100% efficacious, there will always be a few parasites surviving and passing their genes on to the next generation. In a situation with low or even no refugia, all eggs being passed in the feces after deworming will be progeny of resistant parasites mating in the intestinal tract. However, if some of the horses in the herd were left untreated and/or treatments were carried out under circumstances with eggs and larvae present on pasture, then the few resistant worms surviving treatment would be diluted out by the many nonresistant worms, eggs, and larvae, and the progression of resistance in the parasite population would be much slower. The role of parasite refugia in the development of anthelmintic resistance was initially shown in a sheep study,16 and recent work with a combination of computer simulations and field studies with ruminants has provided solid evidence behind the concept.17–19 There have been no such studies performed in horses, so this understanding of parasite refugia is mainly extrapolated from the work performed with ruminants. Over the past five decades the emphasis has shifted several times in terms of which parasites are considered the major targets of the control programs. When the first modern paste dewormers were introduced in the 1960s, the large strongyle Strongylus vulgaris was considered the most important threat to equine health and was therefore identified as the main target for the interval dose program.5 As time went on and new anthelmintic drug classes were introduced, this parasite went from being virtually present in all horses to becoming rare.20–21 At the same time, cyathostomins were recognized as major pathogens and were identified as the primary target of parasite control programs.22 More recently, the tapeworm Anoplocephala perfoliata has been associated with an increased risk of specific colic types.23 In addition, Parascaris equorum is identified as the major parasitic pathogen in foals.24–25 These are the four major parasite pathogens, but others can play a role as well. Less important but widely occurring parasites include Strongyloides westeri, Oxyuris equi, and the bot larvae of Gastrophilus spp. Finally, the insect-borne nematodes Thelazia spp., Habronema spp., Draschia spp., and Setaria spp. can occur at significant levels in certain habitats and are included in this chapter. The equine roundworm has gained status as perhaps the most significant parasitic threat to equine health in managed horse populations. The major reason for this shift of emphasis is the high levels of ivermectin and moxidectin resistance observed worldwide (see Table 49-1). P. equorum is the primary parasite in foals until about the time of weaning. At the age of approximately 6 to 12 months, most horses clear infection with this parasite and the strongyles become the predominating parasite group instead. Equine ascarids are ubiquitous in foaling operations, although prevalence differences can be observed between individual farms.26–27 Patent infection is not uncommon in adult horses, particularly broodmares that are highly exposed to infection. The life cycle is classic ascarid. Eggs are perceived to be highly resilient to environmental influences and can allegedly survive in the environment for several years. Work with eggs of the pig roundworm Ascaris suum has substantiated this, but little work has been done with P. equorum eggs. The infective stage is the embryonated egg, which hatches in the stomach and small intestine of the horse. Larvae then penetrate the mucosal lining and migrate the classical hepatotracheal route. Passing through the lungs has been associated with airway symptoms in young foals,28 but the major pathogenic role is played in the small intestine. Here, the parasites compete with their hosts for nutrients, which can result in ill-thrift, weight loss, rough hair coat, and pot-bellied appearance.25,28 The major clinical impact is associated with verminous small intestinal impactions.24 As with any type of small intestinal impaction, the prognosis for full recovery is guarded and surgery is often required to relieve the condition. In severe cases, the small intestinal walls will rupture and foals will have to be euthanized. Rather ironically, anthelmintic treatment has been shown to be a significant risk factor for this condition.24 The instant paralysis elicited by most dewormers can cause the worms to aggregate in the lower parts of the jejunum and ileum. Benzimidazole-type drugs are not acting through paralysis of the worms and appear to be less associated with this condition. Given the fact that these drugs have maintained efficacy against roundworms, they appear to be a good first choice for this parasite in most instances. Historically, this group is also referred to as small strongyles. It consists of 50 different species belonging to 14 different genera.29 However, some of these species and genera have been described infecting other equids than horses, such as donkeys and zebras. Horses are always infected by multiple species, and currently there is limited knowledge about the pathogenic role of individual cyathostomin species. Cyathostomins are truly ubiquitous, and all grazing horses are exposed to infection. The cyathostomins all follow the same basic strongyle life cycle. Strongyle eggs are passed in the feces, where they will hatch, and larvae will develop through the first (L1) and second (L2) stage until they finally reach the third infective stage (L3). Hatching and subsequent larval development is highly temperature dependent. At temperatures below 6° C, eggs do not hatch but remain viable. Hatching and larval development occur at increasing rates up to about 40° C. At temperatures above this level, eggs and larvae quickly die out. Optimal conditions for development of eggs and larvae are in the temperature range of 25-33° C, where the L3 is reached within 3 to 4 days. In comparison, this will take several weeks at around 10° C. When molting from L2 to L3, the larvae retain their L2 cuticle on the outside of the new sheath. This makes the L3 particularly resistant to environmental influences such as desiccation and freezing. Thus, for this stage, the widespread notion of the “killing frost” is a myth. In addition, intact fecal balls provide excellent protection for larvae, enabling them to further withstand desiccation and freezing temperatures well.30 Due to the double-layered cuticle, the L3 cannot uptake feed and thus lives on carbohydrates and lipids stored in its cells. The more active these larvae are, the shorter they live. Because larval activity is highly temperature dependent, it can be deduced that the higher the temperature, the shorter the survival. Dry conditions as achieved with freezing or desiccation limit larval movement and therefore facilitate their survival. However, repeated cycles of alternation between frost and thaw have a deleterious effect on most free-living stages of strongyles, although this can be mitigated by snow cover, which tends to stabilize temperatures right at the freezing point, which is optimal for survival of eggs and L3s.30 Inside the horse, the L3s exsheath in the stomach and subsequently reach the colon and cecum. Here, they enter the glands of Lieberkühn and penetrate cells at the base. From here, some species appear to penetrate deeper into the submucosa, while others remain in the mucosa. A fibrous capsule is formed around each larva, and a fluid-filled cyst is formed. Larvae can remain encysted for several years,9 but encystment appears to be a strategy for the parasites to make it through the winter, where conditions on pasture are unfavorable for parasite transmission. Interestingly, in warmer climates, encystment appears to occur over the hot summers. Horses can harbor several hundred thousand encysted cyathostomin larvae without showing any sign of discomfort. The major clinical consequences are associated with the process of excystment, where the L4 penetrate their cysts and migrate the short distance back to the intestinal lumen. This causes a small local inflammatory reaction around the vacated cyst. In rare cases, synchronous emergence of large numbers of larvae from their cysts can cause a severe generalized acute typhlocolitis, termed larval cyathostominosis.22 This is characterized by profuse watery diarrhea and pronounced dehydration, as well as protein loss and ventral edema. This acute condition is associated with a case fatality rate of 50%.22 Identified risk factors for larval cyathostominosis include horses aged 1 to 4 years, anthelmintic treatment within 14 days prior, and late fall, winter, or early spring in temperate climates.31 The luminal worm burden appears to elicit some sort of a repressive signal, which prevents the encysted stages from resuming development. If the luminal burden is removed by anthelmintic treatment, the repressive signal disappears abruptly and a synchronous emergence is triggered. Treatment of acute larval cyathostominosis involves intensive fluid therapy, which can be combined with antiinflammatory drugs and antibiotics. The anthelmintic drug of choice is a single dose of moxidectin (400 µg/kg orally [PO]) because of its efficacy against encysted larvae. The 5-day elevated dose of fenbendazole (10 mg/kg PO) is also labeled for treatment of encysted cyathostomins, but the wide occurrence of fenbendazole resistance in cyathostomins makes that regimen unlikely to have maintained full efficacy once resistance has been documented to the single dose.4,32 Chronic cases of larval cyathostominosis also occur. These are typically characterized by weight loss and loose feces or intermittent diarrhea, often over an extended period of time. Plasma protein and albumin levels can be lowered, and ventral abdominal edema can be observed. Horses usually recover, although it may take time for them to regain full body condition. Three species of tapeworm infect horses: Anoplocephala perfoliata, Anoplocephala magna, and Anoplocephaloides (s. Paranoplocephala) mamillana. Of these, A. perfoliata is by far the most common, and it is the only species that has been associated with clinical disease. Being a cestode, the life cycle involves intermediate hosts, which are oribatid mites that feed on the organic material present in the feces. During this process, the mites can ingest tapeworm eggs present in the feces. Inside the mite, eggs develop to infective cysticercoids over a period of approximately 2 to 4 months. Horses inadvertently ingest these mites during grazing. In the gastrointestinal tract, the cysticercoids are liberated from the mites and the scolexes attach to the mucosal lining around the ileocecal valve in the cecum. Prevalences of A. perfoliata have been reported to vary widely between farms and regions, often ranging from 20% to 80% of horses.33 Prevalence appears to be habitat dependent, and it is likely associated with conditions favoring survival of the oribatid mites. However, the most significant factor appears to be access to pasture. The longer the time spent on pasture, the higher the tapeworm exposure seems to become.34 Horses younger than 2 and older than 15 years of age have been reported to have significantly larger Anoplocephala burdens than horses in between these ages.35 Although this could be explained by the assumed incomplete levels of immunity in these age groups, it is equally likely that they simply spend more time on pasture. Tapeworm burdens appear to accumulate through the grazing season to reach their highest in the fall and winter.36–41 Tapeworm infection has been associated with certain types of colic in the horse. Numerous case studies have illustrated an association of A. perfoliata and ileocecal intussusceptions, which are sometimes complicated by intestinal rupture.42–43 One epidemiologic study identified A. perfoliata as significantly associated with ileal impactions and spasmodic colic.23 A Canadian study attempted to reproduce these findings but was unable to find any associations between tapeworms and colic.34 Taken together, these studies indicate that tapeworms may play a role in the development of colic, but this is largely confounded by the multifactorial nature of colics and regional differences in climate and management. Two anthelmintics are labeled for treatment of equine tapeworms: praziquantel and pyrantel pamoate. Praziquantel is a sole tapeworm drug that has no efficacy against nematodes. For this reason, it is most often formulated in combination with ivermectin or moxidectin, although it exists in a stand-alone formulation for horses in Europe.36 Pyrantel, on the other hand, is widely acting against luminal stages of nematodes and cestodes. At the dosage labeled for nematode treatment (6.6 mg/kg PO), the efficacy of pyrantel pamoate is greater than 80% against A. perfoliata.44 A double dosage of pyrantel pamoate (13.2 mg/kg) is labeled for tapeworm treatment, and the efficacy of this has been found to be greater than 95%.45 The strongylin group comprises the three Strongylus species: S. vulgaris, S. edentatus, and S. equinus, as well as Triodontophorus spp., Craterostomum acuticaudatum, and Oesophagodontus robustus. As mentioned earlier in this chapter, large strongyles have generally become rare in managed equine establishments. The most common of these species is probably Triodontophorus spp., but the Strongylus species, particularly S. vulgaris, are considered the most pathogenic of helminths infecting the horse. The development of the external stages of the strongylins is virtually identical to what has already been described for the cyathostomins. Inside the horse, larval migrations are widely different. The three Strongylus species all have PPPs of 6 months and above, and they spend several months migrating in various tissues and organs of the horse. Major pathologic lesions have been ascribed to these migrations, whereas the adult worms are found to do little harm to their hosts. The lesions caused by migrating stages of S. vulgaris are classic in equine parasitology and well described.46–49 Third-stage larvae (L3) invade the mucosa of the small intestine, cecum, and colon. They then molt to L4 before they enter local arterioles. Inside these, they migrate beneath the endothelium toward the root of the cranial mesenteric artery, leaving characteristic fibrous tracts to be seen on the intimal surface. Upon reaching their destination about 14 days post infection, they enter the lumen of the vessel and remain there, embedded in thrombus masses. Here, they grow in size and molt to L5, while causing a pronounced verminous endarteritis with roughened intima, fibrosis of arterial walls, and increased diameter of the vessels. After about 4 months, the L5s are transported by the bloodstream to the walls of the ventral colon and cecum, where pus-filled nodules are formed around them in the submucosa. These nodules eventually open into the intestinal lumen, and the young adult worms emerge. After another 6 weeks, the worms become sexually mature and start shedding eggs. Although the lesions are pronounced, they are relatively rarely accompanied by disease. A classical syndrome of thromboembolic colic, in which thrombi detach from the arteritis lesions and are carried distally until they reach a terminal branch sufficiently small to become occluded, has been described.46 This causes ischemia and infarction of intestinal segments, which is painful to the horse. Larvae of S. edentatus migrate via the portal system to the liver, where they molt to the fourth stage within the parenchyma. They migrate within the liver and then migrate subperitoneally and retroperitoneally via the hepatorenal ligament to the adipose tissue in the abdominal walls. Larvae can also be found located in the perirenal fat tissue. Here, larvae eventually molt to L5 and migrate back to the intestinal walls. Described lesions include a filamentous peritonitis, especially around the liver and diaphragm, as well as hemorrhagic and inflammatory lesions in the abdominal wall.50 The third Strongylus species, S. equinus, is probably the rarest, at least in managed horses in the northern hemisphere, where it is hardly encountered at all. As opposed to S. edentatus, it migrates within the peritoneal cavity and passes through the pancreas, where it can cause significant damage.51 Despite their obvious pathologic impact, neither S. edentatus nor S. equinus have been associated with defined clinical syndromes. None of the strongylins has been reported resistant to any of the anthelmintic drug formulations available. Treatment efficacies against S. edentatus appear to be more variable than in the other species,52 but this seems to always have been the case and can thus not be concluded to be resistance. Other parasites frequently infect equines but are generally considered of lower importance. For completion, they are briefly covered in the following. Strongyloides westeri, or the equine threadworm, parasitizes the small intestine of suckling foals. This parasite is remarkable because it can maintain a full life cycle outside its host, and only female worms seem to infect the foals. Infection occurs via three possible routes: skin penetration by third-stage larvae, ingestion of these larvae from the environment, or lactogenic transmission from the mare.53 Infection usually occurs within the first weeks of life, and infected foals are usually asymptomatic. However, one study reported an association between diarrhea and high Strongyloides egg counts (>2000 EPG),54 and another study described a “frenzy” syndrome in foals exposed to apparent percutaneous penetration of L3 from the environment.55 Because of S. westeri, it has become a widely used practice to deworm mares at or just before foaling. As a result, the prevalence of this parasite has been reduced to low levels in managed horse populations.56 Oxyuris equi, the equine pinworm, is widely prevalent, but eggs are rarely encountered in fecal samples because they are laid outside the digestive tract. Adult worms live in the descending colon and rectum. The female worm protrudes from the anus and deposits her eggs in patches on the perianal skin. When these egg patches dry up, they become itchy to the horse, and they begin rubbing their tails against various objects. This serves as an excellent means to spread eggs in the environment. The infective stage is the embryonated egg, which is inadvertently ingested from the contaminated environment. Besides the tail rubbing, O. equi does not cause disease or discomfort to the horse, and there is no justification for routinely targeting this parasite in the parasite control program. Recent observations made by veterinary practitioners have led to speculations whether populations of O. equi have developed resistance to ivermectin,57 but this has not been verified in research studies. Gasterophilus spp. are the widely occurring botflies, whose larval stages overwinter in the intestinal tract and then pass in the feces during the spring. They then pupate in the loose soil for a couple of months until the adult flies emerge. The two most common species are G. intestinalis and G. nasalis. Eggs are glued onto the haircoat, and larvae make their way into the oral cavity by either crawling on the skin (G. nasalis) or when the horse grooms itself or another herd member (G. intestinalis). In the mouth, the larvae spend several weeks burrowing into the tongue and subsequently spending time in the interdental spaces before reaching the stomach.58 In the stomach, G. intestinalis attaches to the mucosal lining by the margo plicatus, whereas G. nasalis can be found in the gastric fundus and the duodenum. Characteristic lesions have been described at the attachment site,59 but these have not been consistently associated with disease. With the emergence of equine dentistry over the past decades, the lesions in the oral cavity are gaining more attention. It remains possible that Gasterophilus spp. can cause oral discomfort, but this has not been substantiated in research studies. The equine eye worm, Thelazia lacrymalis, is transmitted by muscid flies and can be found within the conjunctival sac of horses, where it generally appears to do little or no harm to the eye. However, abscess formation and inflammation of the lacrimal glands have been described.60 Prevalence rates have been reported in the range of 20% to 42% in managed horses.61–62 The group of stomach worms comprises Habronema spp., Draschia spp., and Trichostrongylus axei. Habronema and Draschia spp. have similar life cycles. Like Thelazia spp., they are both transmitted by muscid flies. The flies acquire the infective larvae while feeding on the feces. Horses get infected by accidentally ingesting infected flies. Here, the larvae eventually reach the stomach, where Habronema larvae apparently cause no gross lesions, while adult specimens of Draschia spp. can be found embedded in large tumor-like fibrous masses around the margo plicatus.63 Common to both these species is that the larvae can be deposited by the flies in or near wounds or mucocutaneous junctions. For the parasite, this represents a dead-end pathway, from which the life cycle cannot be completed.64–65 When larvae are deposited in this manner, they cause a condition usually referred to as cutaneous habronemiasis/draschiasis or summer sores. This is characterized by persistent, eosinophilic granulomatous lesions with eosinophilia and fibrosis. A rare, pulmonary form of habronemiasis has been described as well.66 Anthelmintic resistance has not yet been reported in Habronema or Draschia spp., but several veterinary practitioners from subtropical and tropical regions report that skin lesions consistent with cutaneous habronemiasis do not resolve on treatment with a macrocyclic lactone (ivermectin or moxidectin). The prevalence of Draschia spp., on the other hand, seems to have decreased dramatically after the introduction of ivermectin.67 Trichostrongylus axei possesses the rare capacity of infecting both ruminants and monogastrics such as pigs and horses. The external part of the life cycle is similar to that of the strongyle parasites described in this chapter. Inside the horse, it develops within the gastric glands, where it can cause hypertrophy of the mucosa.68 In horses, T. axei has not been found to affect pH or levels of plasma pepsinogen.69 The parasite is a rare finding in horses, but prevalence has been found to increase when horses are co-grazed with sheep.70–71 Setaria and Onchocerca spp. are filarial nematodes infecting horses. Infective L3 larvae are transmitted by blood-sucking arthropods feeding on the horses. The larvae then migrate to their predilection site, which is the connective tissues (Onchocerca spp.), and the abdominal cavity (Setaria equina). Here, they develop into adult worms, which then release microfilariae into the bloodstream. These microfilariae, in turn, will enter the blood-feeding arthropods, and the cycle is complete. The intermediate hosts for Onchocerca spp. are biting midges (Culicoides spp.) and black flies (Simulium spp.), whereas it is mosquitoes for Setaria equina. The latter parasite is regarded as nonpathogenic, while Onchocerca spp. has been associated with pronounced skin reactions. Microfilariae of Onchocerca cervicalis tend to congregate in certain regions of the body, including the ventral midline and face, where they are ingested when Culicoides feed in these regions.72 This results in a verminous dermatitis characterized by intense pruritus.73 Lesions can be effectively treated with macrocyclic lactones.74 However, there is no known therapy to eliminate adult O. cervicalis from the connective tissue around the ligamentum nuchae of horses. Therefore, infected horses will need periodic retreatment with ivermectin or moxidectin to prevent or control recurrence of clinical signs.75–77 Treatment of the skin-dwelling microfilariae has been associated with an inflammatory reaction to the dying parasites,78 which can be confused with the widely occurring Culicoides hypersensitivity (summer eczema, sweet itch). Adult Parafilaria multipapillosa is another filarial nematode that occurs in subcutaneous and intermuscular connective tissue of horses outside North America. Nodules form in the overlying skin and may rupture and bleed or leak tissue fluids. This condition is often referred to as “summer bleeding,”79 not to be confused by the “summer sores” described with cutaneous habronemiasis/draschiasis. First-stage larvae are present in the exudate from bleeding lesions and are ingested by feeding horn flies (Haematobia irritans). Larvae develop to the infective third stage within the fly and are transferred to horses when flies feed on lachrymal secretions or skin wounds. The larvae then migrate in the subcutaneous tissues and develop to the adult stage within a year. Eggs and microfilariae can readily be identified in smears taken from lesion exudates.80 Little is known about anthelmintic efficacy, but lesions have been observed to recur after treatment with macrocyclic lactones. The equine lungworm, Dictyocaulus arnfieldi, will be covered in the lungworm section in this chapter. Halicephalobus gingivalis is a free-living nematode belonging to the order Rhabditida. It is widely present in the soil and humus but possesses a capacity to also invade living tissues. Infection appears to occur through soil-contaminated wounds or nasal mucosal membranes with apparent predilection in the head region,81–84 but penile infection has also been described.85 Inside the host, the nematode reproduces tremendously and the larvae invade deeper into the tissues. Larvae appear to have a special affinity for the central nervous system (CNS) and the kidneys.83 Lesions are multifocal, eosinophilic, and pyogranulomatous. Once the CNS is affected, symptoms often slowly progress into a grave state. Symptoms include blindness, ataxia, loss of proprioception, head pressing, coma, recumbency, and death.83,86–87 Treatment options are limited. Surgical debulking can be combined with repeated high oral doses of fenbendazole (50 mg/kg) or ivermectin 0.55 mg/kg),88 but the overwhelming majority of published cases had a fatal outcome. Similarly, Halicephalobus spp. infection has been reported to be extremely rare but fatal in humans.89–91
Parasite Control Programs

Equine Parasitic Disease
Anthelmintic Resistance
Egg Reappearance Period
Prepatent Period
Species
Prepatent Period
References
Cyathostomins
2-3 mo*
113
Parascaris equorum
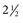 -3 mo
-3 mo
114
Anoplocephala perfoliata
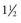 -4 mo
-4 mo
115
Strongylus edentates
11-12 mo
115
Strongylus equinus
115
Strongylus vulgaris
6-7 mo
112
Parasite Distributions
Parasite Refugia
Important Parasites Infecting Horses
Parascaris equorum
Cyathostomins
Tapeworms
Strongylins (Large Strongyles)
Other Parasites
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Parasite Control Programs
Chapter 49