Chapter 20 Otoscopy
Video Otoscopy
Indications
Additionally, in patients with chronic otitis externa, the predisposing factors, primary causes, and perpetuating factors must be identified to control the ear disease. Primary causes of otitis externa are those that result in ear disease in the absence of other factors. Primary causes include parasitic diseases, hypersensitivity disorders, foreign bodies, disorders of keratinization, autoimmune disease, and neoplasia.
Indications for the Use of Video Otoscopy in the Examination Room
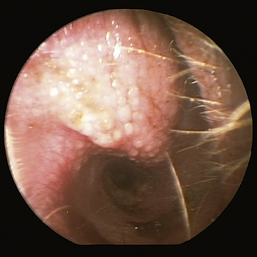
The normal vertical and horizontal ear canal should be pink, and in most animals, the cartilage is flexible; thus the otoendoscope should advance easily down the canal. There can be small amounts of wax in the ear canal. In animals with otitis externa, the changes detected in the ear canal are related to the severity of the ear disease. The first changes noted in the ear canals in cases of acute otitis externa include erythema and glandular hyperplasia (Figure 20-1). The amount of exudate in the ear canal is variable and should be documented, as should its color and consistency. A swab sample from each ear should be obtained for cytologic evaluation. If numerous rod bacteria are noted cytologically or the infection has worsened since the last visit, then a bacterial C/S is indicated. In more severe cases of otitis externa, glandular hyperplasia progresses, resulting in stenosis of the canal, sometimes to the point where the otoendoscope can no longer be advanced into the vertical ear canal. The degree of stenosis and hyperplasia should also be documented. Ulcerations may also be noted, especially in ears secondarily infected with P. aeruginosa. Parasites such as Otodectes cynotis may be visualized rapidly moving about in the ear canal with the video otoscope and are usually found in conjunction with dark brown ceruminous (“coffee ground”) exudate. On occasion, masses or foreign bodies may be seen in the ear canal during the video otoscopic examination.
Indications for the Use of Video Otoscopy for Deep Ear Flushing, Myringotomy, Biopsy, and Foreign Body Retrieval
Instrument Disinfection
After the video otoscope and any associated equipment (e.g., curette, forceps, or dual-port adapter) is used, it is important to clean and disinfect the equipment. The clinician can remove exudate in the port of the otoendoscope by passing the manufacturer’s cleaning brush in and out of the port. The otoendoscope along with any equipment used during the procedure is first cleaned with an enzymatic cleaner. The soaking time will be indicated by the manufacturer of the cleaner, but soaking times should never exceed those recommended by the manufacturer of the otoendoscope to prevent damage. The otoendoscope and equipment are scrubbed with brushes and towels and then transferred to a distilled water bath to rinse off the enzymatic cleaner. After the otoendoscope and equipment are cleaned, they are disinfected by soaking in glutaraldehyde for the length of time indicated by the manufacturer of the otoendoscope. Alternatively, the curette and forceps may be gas sterilized. Some otoendoscopes may also be gas sterilized or autoclaved.
Otic Anatomy
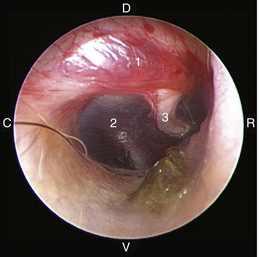
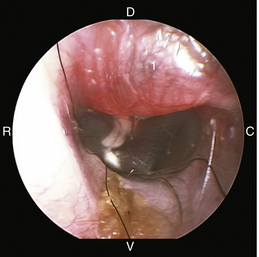
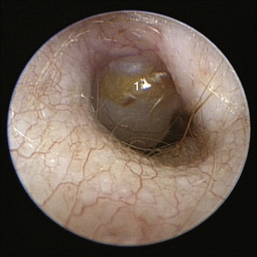
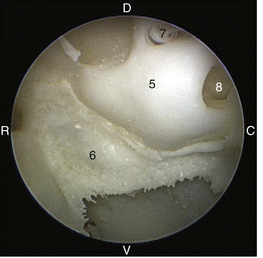
The middle ear consists of an air-filled tympanic cavity, three auditory ossicles, and the tympanic membrane. The tympanic membrane is located at a 45-degree angle in relation to the central axis of the horizontal part of the external ear canal. The tympanic membrane is a semitransparent membrane that separates the external ear canal from the middle ear, is thin in the center and thicker at the periphery, and is divided into two sections, the small upper pars flaccida and the larger lower pars tensa (Figure 20-2). The pars flaccida is the pink, small, loosely attached region forming the upper quadrant of the tympanic membrane that contains small blood vessels. The pars flaccida is usually flat; however, even in the healthy ear one may identify a bulging pars flaccida (Figure 20-3). The exception is the Cavalier King Charles Spaniel, in which a bulging pars flaccida may be indicative of a disease known as primary secretory otitis media (PSOM) (Figure 20-4).
The pars tensa, the thin, tough, gray structure with radiating strands, occupies the remainder of the membrane. The pars tensa is attached to the osseous ring of the external acoustic meatus. The manubrium of the malleus attaches to the medial surface of the tympanic membrane. The outline of the manubrium of the malleus, the stria mallearis, may be visualized when the tympanic membrane is viewed externally. The pars tensa has a concave shape when viewed externally because of the tension applied to the internal surface of the membrane, where the manubrium of the malleus is attached. The point of greatest depression is called the umbo.
The tympanic cavity consists of a small epitympanic recess, a large ventral bulla, and the tympanic bulla proper. In the dog, there is an incomplete bony septum, the bulla septum, which allows communication between the tympanic bulla proper and the ventral tympanic bulla. On the medial wall of the tympanic cavity, there is a bony eminence, the promontory, which houses the cochlea, and lies opposite the tympanic membrane medial to the epitympanic recess. At the caudolateral portion of the promontory, a foramen called the cochlear (round) window is located. The cochlear window is covered by a thin membrane that oscillates to dissipate the vibratory energy of the perilymph in the scala tympani. The vestibular (oval) window lies on the dorsolateral surface of the promontory immediately adjacent to the pars flaccida (Figure 20-5). It is covered by a thin diaphragm. The footplate of the stapes is attached to the diaphragm over the vestibular window. When flushing the middle ear, one must be very careful to avoid damaging the promontory or the cochlear window and to avoid damaging the inner ear, causing vestibular disturbances or deafness.
Procedure for Deep Ear Flush with the Video Otoscope
The video otoscope is an invaluable tool for evaluating and flushing the middle ear of an anesthetized patient with otitis media. I would encourage standardizing the orientation of the video otoscope relative to the animal when utilizing the video otoscope in the anesthetized patient. That way, the surgeon avoids becoming disoriented when the anatomy of the ear is abnormal. Next, make sure that the monitor is easily visible while examining the ear, which means that it is directly in front or to the left or right. It is very awkward for the surgeon to try to view the monitor over his or her shoulder.
After the external ear canal has been soaked with a ceruminolytic agent or ear cleaner, the external ear canal is flushed with warm sterile isotonic saline with the use of a bulb syringe to remove large debris and exudate. This is followed by flushing with warm sterile isotonic saline with the use of a handheld otoscope with an 8F polypropylene urinary catheter attached to a 12-mL syringe passed through an otoscopic cone. An 8F red rubber feeding tube may be cut to size and used to flush the external ear canal. External flushing devices, such as the Vet Pump 2, available from the manufacturer of the video otoscope, may be used in place of the manual flushing described above. Once the ear is clean, the tympanic membrane is evaluated with the video otoscope and images are taken for documentation. If the tympanic membrane is ruptured or torn, as previously stated, it is important to flush copious amounts of sterile saline into the ear canal to remove the ceruminolytic agent. In addition, once a ruptured or torn tympanic membrane has been identified, do not infuse the ceruminolytic agent into the bulla. Once the exudate has been removed from the external ear canal, I attach the dual-port adapter to the port on the otoendoscope. The dual-port adapter is used to perform passive suctioning and flushing of the external ear canal, which expands the ear canals, further magnifies the ear, prevents lens fogging, and continues to clean the ear. A warmed bag of sterile saline is attached to a Venoset on one port of the dual-port adapter, and a suction hose is attached to the other port. A tear or rupture can be identified by bubbles coming from the tympanic membrane. Once the patency of the tympanic membrane has been determined, I will remove the saline from the ear canal and evaluate the tympanic membrane with the otoendoscope because saline in the ear canal can sometimes cause the tympanic membrane to look opaque (which would be considered abnormal) when it is not. If the tympanic membrane is ruptured, then the patient has otitis media. A myringotomy does not need to be performed, and samples from the middle ear for cytologic and culture analysis can be obtained through the tear in the tympanic membrane. I find it helpful to have technical assistance in obtaining these samples. I will turn the otoendoscope to where the port is ventral, without moving the camera. This allows easy access to the port and keeps the instruments aimed ventrally so that the auditory ossicles and promontory are avoided. I will have a technician stand behind the patient’s head and hold the ear in one hand and the video otoscope in the other. This allows me to have my hands free to flush the middle ear (Figure 20-6). An open-end 3½F Tomcat catheter attached to a 12-mL syringe is placed through the port of the otoendoscope. Saline is flushed into the middle ear cavity and aspirated back for culture, and a second sample is flushed into the middle ear and aspirated back for cytologic analysis. After sample retrieval, the middle ear is flushed with warm sterile saline through the open-ended Tomcat catheter. I will flush and aspirate back 1 mL of sterile saline at a time and will finish when the saline flushed from the middle ear cavity is clear.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree