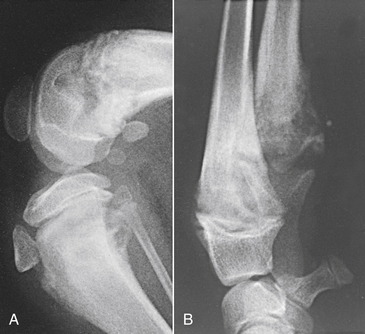
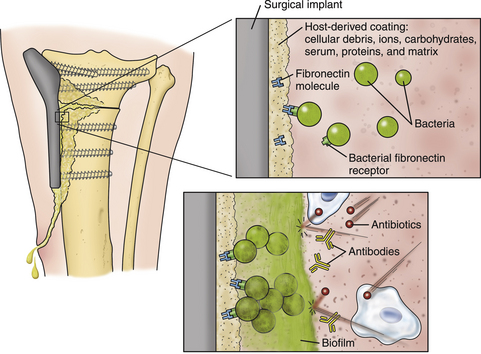
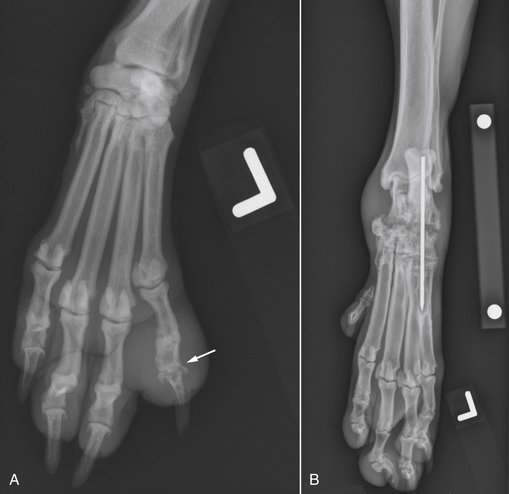
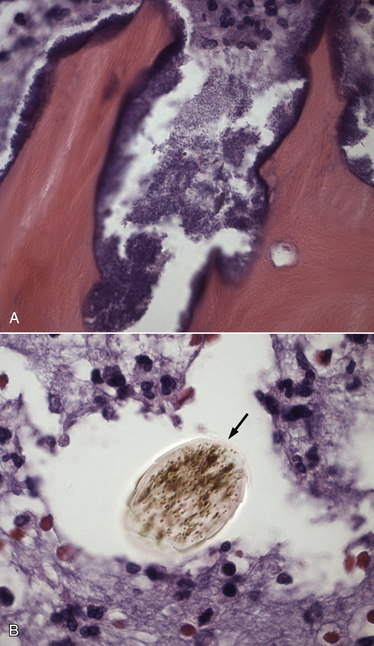
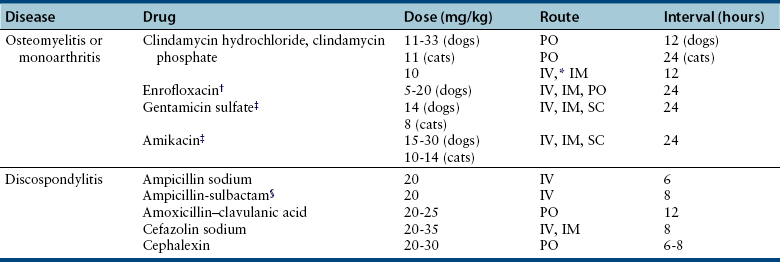
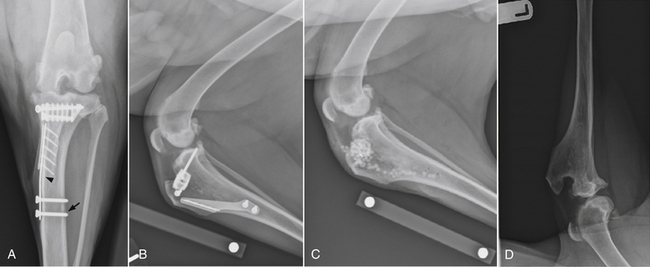
Chapter 85 Osteomyelitis, discospondylitis, and arthritis can result from (1) hematogenous spread of bacteria or fungi to these sites, or (2) from direct introduction of bacteria as a result of penetrating wounds or surgery. When fungi are involved, molds such as Aspergillus spp. or Paecilomyces spp. are more likely than yeasts to cause discospondylitis; Candida has rarely been associated with discospondylitis in dogs.1 In contrast, osteomyelitis can be caused by opportunistic molds or dimorphic fungi, especially Coccidioides spp. or Blastomyces dermatitidis. Fungal arthritis is uncommon but has been associated with infection by Coccidioides spp., B. dermatitidis, and Cryptococcus spp. Etiology, Pathogenesis, and Epidemiology Bacterial osteomyelitis most often results from direct introduction of bacteria into wounds adjacent to bone. Bacteria can be introduced into the bone as a result of penetrating foreign bodies, surgical wounds (especially orthopedic surgeries with implant placement), traumatic wounds, bite wounds, or extension of soft tissue infections. Tibial plateau leveling osteotomy (TPLO) surgery for cranial cruciate rupture, one of the most common orthopedic surgical procedures performed, has a postoperative infection rate that ranges from 0% to 7%.2–5 For unknown reasons, the infection rate after TPLO appears to be higher than that after routine “clean” orthopedic surgeries that involve osteotomy (as opposed to fracture repair). In a report that was published before the TPLO procedure was described, the overall infection rate after any orthopedic surgery procedure was 3 of 502 surgeries (<1%).6 In contrast, radiographically detectable osteomyelitis was reported in 14 (7.3%) of 193 dogs that underwent TPLO surgery and was the single most common complication of the procedure.3 In another study, the infection rate after fracture repair was 9 (8%) of 110 surgeries.7 In this study, 20% of the fractures were open. Open fractures were 4 times more likely to be contaminated with bacteria at the time of surgery than the closed fractures, and contaminated wounds were 5 times more likely to develop complications (seroma, drainage, and dehiscence) than those without contamination. Distal radius/ulna and tibial fractures were 10.5 times and 7.5 times more likely to be contaminated than femoral fractures, respectively. Animals with a normal postoperative temperature and a clean surgical wound did not develop postoperative complications. Although gram-positive aerobes such as staphylococci and, to a lesser extent, streptococci predominate as a cause of osteomyelitis in dogs and cats, the organisms present and the bone(s) affected generally reflect the underlying type of injury that led to infection. For example, osteomyelitis that follows surgical site infection is often caused by Staphylococcus pseudintermedius, a common commensal of canine skin (Table 85-1). Staphylococcus aureus and gram-negative bacterial infections can also cause orthopedic surgical site infections.5 In contrast, anaerobes and aerobic gram-positive bacteria (staphylococci, enterococci, streptococci) or gram-negative bacteria (Pseudomonas, E. coli, Klebsiella pneumoniae, Proteus, Pasteurella) predominate in osteomyelitis that results from bite wound infections, tooth root abscesses, or penetrating foreign bodies.8,9 Such mixed bacterial infections are also common in dogs with digital osteomyelitis. TABLE 85-1 Bacteria Isolated from 31 Dogs with Surgical Site Infections after Tibial Plateau Leveling Operations at the University of California, Davis, Veterinary Medical Teaching Hospital NA, Not applicable; ND, not done. ∗Resistant to three or more classes of antimicrobial drugs. †One of 2 E. coli and the Proteus isolate did not undergo susceptibility testing because isolation was performed at necropsy. Osteomyelitis secondary to bacteremia, also known as hematogenous osteomyelitis, is very rare and usually affects young animals. The metaphyseal regions are often involved, sometimes with extension to the epiphyses and joints (Figure 85-1). The pathogenesis remains obscure. It has been suggested that discontinuous endothelium in the primary spongiosa may predispose to extravasation of erythrocytes and bacteria in this region.10 In humans, blunt trauma appears to contribute to development of focal childhood osteomyelitis, and it has been hypothesized that the periosteum, rather than the metaphysis, may initially be involved.11 Sternal and vertebral osteomyelitis can also result from hematogenous spread of bacteria. Osteomyelitis occasionally also results from hematogenous spread of fastidious bacteria such as Brucella, Bartonella, Mycobacterium, and Nocardia spp. FIGURE 85-1 Polyostotic metaphyseal osteomyelitis in a Border collie puppy that had persistent neutropenia. A, Disruption of metaphyseal architecture of distal femur and proximal tibia, with irregular areas of radiolucency and sclerosis and some adjacent periosteal new bone. B, Similar changes are evident in the distal radius and ulna. (From Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine. 7th ed. St. Louis, MO: Saunders; 2010.) The development of bacterial osteomyelitis requires the presence not only of bacteria, but also of compromised bone. Bone necrosis can result from trauma (i.e., fracture), surgery, or underlying bone neoplasia. Bacteria adhere to fibronectin, a connective tissue glycoprotein, through the use of a variety of adhesins. The presence of foreign material promotes persistence of infection in the form of biofilm (Figure 85-2). Within biofilm, bacteria evade opsonization and phagocytosis and are protected from the effects of antimicrobial drugs. Bone sequestra can also become colonized by bacteria, which evade host defenses in the absence of adequate blood supply. Loose implants and fracture instability contribute to bone resorption, compromised blood supply, and persistent infection.10 Some implant types are more apt to be colonized by bacteria. In one study, Slocum TPLO plates were more likely to be removed than other types of TPLO plates because of local bacterial infection and clinical signs of inflammation.12 FIGURE 85-2 Pathogenesis of osteomyelitis. Necrotic bone is colonized by bacteria, which adhere to fibronectin and in the presence of foreign material (in this case, an implant) can form biofilm. Biofilm consists of bacterial aggregates embedded in a matrix of exopolysaccharide (bacterial slime). The biofilm protects the bacteria from antibodies, phagocytosis, and antimicrobial drugs. Inflammatory cells recruited to the site of infection release enzymes that cause additional bone necrosis, with destruction of cancellous and cortical bone and abscessation of associated soft tissue. The end result is focal swelling, pain, draining skin lesions, and lameness. Small draining skin lesions often represent the “tip of the iceberg”, with extensive bone involvement hidden beneath the skin (Figure 85-2). The presence of anaerobes can be associated with a foul odor or gas production. Fever, inappetence, lethargy, and leukocytosis are occasionally present in animals with acute osteomyelitis but are often absent in chronic osteomyelitis. Occasionally, infection reactivates in dogs years after surgery and implant placement. In human patients, latent periods between infection and development of osteomyelitis can last many decades.13 Radiographic changes in dogs and cats with osteomyelitis vary considerably in extent and character. In some dogs and cats, only soft tissue swelling may be evident. Plain radiography is insensitive and can be poorly specific for diagnosis of osteomyelitis, and interpretation can vary from one board-certified radiologist to another. In one study, plain radiographic findings were interpreted as osteomyelitis by two radiologists in only 5 of 8 dogs with experimentally induced osteomyelitis.14 One radiologist diagnosed osteomyelitis in only 3 of the 8 dogs, but that radiologist was less likely than the other radiologists to diagnose osteomyelitis when infection was absent. When radiographic bony changes are evident, they consist of periosteal proliferation, which may be smooth or speculated; focal osteopenia with cortical resorption or thinning; loss of trabecular markings; lucency around implants such as plates or screws; and sequestration (Figure 85-3). Pathologic fractures may be evident in dogs with fungal osteomyelitis. Scintigraphy with technetium-99m ciprofloxacin has also been investigated for diagnosis of osteomyelitis in a rabbit model.15 False negatives and false positives were common early (4 weeks) in the course of healing, but sensitivity increased to 75% to 100% later in the course of healing, with specificities that approached 100%. Ultrasound imaging, contrast computed tomography (CT), and MRI may be useful for identification of sequestra, foreign material, and abscess formation. Intravenous administration of ultraparamagnetic iron oxide nanoparticles during MRI, which are taken up by macrophages, has shown promise for early detection of vertebral osteomyelitis and differentiation of osteomyelitis from aseptic inflammation.16 FIGURE 85-3 Radiographic changes in bacterial osteomyelitis. A, Osteomyelitis of the third phalanx of the fifth digit in a 7-year-old female spayed fox terrier with systemic lupus erythematosus that had been treated with prednisone and cyclosporine. There is severe soft tissue swelling, moderate lysis of the distal aspect of the third phalanx, and an irregular cortical margin with loss of the normal P2-P3 joint space (arrow). A Nocardia sp. was isolated from the draining lymph node. B, Radiographs of the left tarsus of a 5-year-old male neutered pit bull terrier with implant-associated osteomyelitis. A partial left tarsal arthrodesis had been performed a year previously after the dog suffered a traumatic injury. Draining tracts then developed in association with the screw heads, and treatment with cephalexin was unsuccessful. There is severe irregular bone lysis on both sides of the proximal intertarsal joint. A surgical pin extends from the proximal calcaneus through the tarsal joint to the level of the mid-diaphysis of the 5th metatarsal bone. There is a region of lucency that surrounds the tip of the surgical pin within the 5th metatarsal bone. The cortex of the 4th metatarsal bone adjacent to the pin is thickened with a slightly irregular periosteal reaction. The first phalanx of the first digit is incomplete (likely congenital). There is marked soft tissue swelling associated with the tarsus. A methicillin-susceptible Staphylococcus aureus was cultured from the implant at surgery to remove the pin. Tobramycin-impregnated calcium sulfate beads were used to fill the pin tract. Pathologic findings in osteomyelitis depend on the causative agent and chronicity of infection. Inflammatory cell infiltrates may range from suppurative (acute osteomyelitis) to pyogranulomatous or lymphoplasmacytic. The neutrophilic infiltrate in dogs with acute osteomyelitis may be accompanied by the presence of fibrin, free red blood cells, and necrotic debris. There may be evidence of bone resorption with multinucleated osteoclasts and new bone and fibrous tissue formation. In some cases, intralesional bacteria or fragments of foreign material (such as plant material) are observed (Figure 85-4). FIGURE 85-4 Histopathology from the right pelvic fourth digit of a 3-year-old intact male Labrador retriever with an open articular fracture and chronic suppurative bacterial osteomyelitis. A, Large numbers of bacteria are adhered to bone fragments (eosinophilic material) with an associated neutrophilic inflammatory response. B, In other fields, intralesional plant material was visible (arrow). Treatment recommendations for focal osteomyelitis secondary to trauma, surgery, or foreign body penetration depends on the severity and chronicity of disease, the location of osteomyelitis, the presence or absence of foreign material, and the extent of blood supply to the bone. In animals with fractures, early detection and treatment of osteomyelitis is critical to prevent extensive bone necrosis and nonunion. Antimicrobial drug treatment alone is usually not sufficient to resolve chronic bacterial osteomyelitis, because sequestra and biofilm are avascular and are not effectively penetrated by antimicrobial drugs. In general, treatment requires debridement, removal of sequestra and foreign material (such as implants), dead space removal, extensive lavage with sterile saline, rigid fracture stabilization, systemic +/- local administration of appropriate antimicrobial drugs, and grafting of bone deficits. Because implants must ultimately be removed to resolve infection, fractures may need stabilization through alternative means, such as external skeletal fixation. If implants are rigidly stable, they should be left in place until bone healing is complete and then they should be removed in a second surgical procedure. Systemic antimicrobial drug therapy (and often regional antibiotic therapy in the form of antimicrobial drug–impregnated beads) is required while the bone heals. In some cases, foci of osteomyelitis may be removed en bloc without the need for further graft placement or stabilization (e.g., digital osteomyelitis). Resected bone should be submitted for histopathology (to confirm the presence of osteomyelitis and identify other processes such as fungal infection or neoplasia) as well as culture and susceptibility. Wounds are left open after debridement if continued debridement is needed via wet-to-dry bandaging. If all nonviable tissue has been removed, closed irrigation systems or vascularized tissue flaps may be used to manage dead space. A sterile dressing should be used to cover the wounds, with daily bandage changes until exudation resolves. If needed, autogenous cancellous bone graft placement is delayed until infection has been controlled and granulation tissue has begun to fill the wound (usually 1 to 2 weeks after initiation of antimicrobial drug treatment).17 When graft placement or fracture stabilization is not possible because of financial concerns or uncontrollable infection, amputation may be necessary. The choice of antimicrobial drugs should be based on the results of aerobic and anaerobic bacterial culture and susceptibility. If the animal is stable, treatment should be withheld until results are available, and medications to manage pain should be administered. If earlier antimicrobial treatment is deemed necessary pending the results of culture and susceptibility, the drug chosen should be based on the history (e.g., postsurgical infection versus bite wound infection) and local prevalence data for the practice (Table 85-2). Clindamycin is a reasonable initial choice for infections that could include susceptible staphylococci and/or anaerobic bacteria and was effective in experimental models of S. aureus osteomyelitis in dogs,18 but a significant proportion of methicillin-resistant staphylococci exhibit clindamycin resistance or inducible clindamycin resistance (see Chapter 35). If gram-negative bacteria or methicillin-resistant staphylococci are suspected, the addition of an aminoglycoside may be necessary if treatment must be instituted before the results of culture and susceptibility become available. However, aminoglycosides penetrate poorly into purulent material, so adequate debridement is essential. Combinations of antibiotics are sometimes required in animals with polymicrobial infections. Systemic antibiotic treatment should be continued for at least 4 to 6 weeks. Longer periods of treatment may be required in some dogs and cats. Antimicrobial drugs should be given parenterally in the hospital for at least the first 3 to 5 days, and for longer if possible. TABLE 85-2 Suggested Initial Antimicrobial Drug Doses for Stable Animals with Bacterial Orthopedic Infections Pending Culture and Susceptibility Results See Chapter 86, Table 86-8, for suggestions for severe sepsis. ∗For IV administration, dilute 1:10 in 0.9% saline and administer over 30-60 minutes. †Addition of fluoroquinolones to clindamycin or a β-lactam is indicated if gram-negative bacteria may be present and methicillin resistance among staphylococci is not prevalent in the region. In cats, other fluoroquinolones such as pradofloxacin or marbofloxacin are preferred because of the risk of retinopathy (see Chapter 8 for doses and contraindications). Use of pradofloxacin has been associated with bone marrow suppression in dogs, especially at high doses. ‡Addition of aminoglycosides to clindamycin or a β-lactam is indicated if gram-negative bacteria and/or methicillin-resistant staphylococci may be present. See Chapter 8 for contraindications. In addition to systemic treatment, antibiotic-impregnated beads can be used to treat chronic osteomyelitis and septic arthritis in dogs.19–21 These are prepared in the operating room by mixing an antimicrobial drug with commercially available cement and setting the cement into beads on a suture through the use of a plastic mold or by hand. The beads are counted and strings of beads are placed at the site of infection, where they deliver high concentrations of drug to the site (e.g., initially 500-fold that of normal serum concentrations with systemic treatment), sometimes for several weeks, without systemic adverse effects.22 The antimicrobial drugs used are generally heat stable, hydrophilic, and have activity against methicillin-resistant staphylococci, such aminoglycosides (gentamicin or tobramycin) or vancomycin. Clindamycin has also been used. Once the antimicrobial drug has diffused from the bead, nonbiodegradable beads such as those composed of polymethylmethacrylate (PMMA) may act as foreign bodies and a nidus for infection, and so a second surgery is usually required for bead removal. Biodegradable materials are also available, and include antibiotic-impregnated collagen, bone graft substitutes such as calcium phosphate or calcium sulfate (plaster of Paris, e.g., Osteoset) beads (Figure 85-5), and a variety of synthetic polymers such as polylactic acid, polylactide-co-glycolides, and cross-linked polydimethylsiloxane.22 Tobramycin-impregnated calcium phosphate beads have been used successfully to treat osteomyelitis in dogs.20 Polymers can be engineered to control the duration and concentration of antibiotic treatment at the site. FIGURE 85-5 Lateral radiographs of the left stifle from a 10-year-old female spayed greyhound–pit bull terrier mix that developed osteomyelitis at the site of a left tibial tuberosity advancement surgery (see Case Example). Anteropalmar (A) and lateral (B) radiographs of the left stifle 13 months after the surgery. The osteotomy site has filled with bone to some extent, but the site is still visible as a result of incomplete union. There was also lucency in the region of the most distal screw (arrow) and thinning of the cortical bone adjacent to the plate (arrowhead). C, The cage was removed by drilling around it, and calcium sulfate (Osteoset) beads were used to fill the defect. D, Radiograph taken 13 months postoperatively showing resolution of osteomyelitis and degradation of the calcium sulfate beads. Although not a substitute for sterile surgical technique and strict hospital infection control measures (see Chapter 11), preoperative antimicrobial drug treatment is an accepted aid for prevention of soft tissue surgical infections and osteomyelitis, especially when orthopedic implants are anticipated. Guidelines published for human medicine state that adequate prophylaxis requires (1) antimicrobial agents with activity against organisms likely to be encountered in the surgical field; (2) timely preoperative antimicrobial drug administration (within 60 minutes of incision); (3) bactericidal concentrations of drug in the serum and tissues during the time that the wound is open; and (4) a duration of 24 hours after surgery.23 Additional intraoperative doses should be given if the procedure lasts longer than two half-lives of the drug being administered (usually at least 2 hours). In practices where the prevalence of postoperative infections with methicillin-resistant staphylococci is low, preoperative treatment with cefazolin may be effective. Where the regional prevalence of skin colonization with methicillin-resistant staphylococci is high (e.g., >10%), preoperative treatment with cefazolin and an aminoglycoside such as amikacin could be used. Prophylactic treatment should never be continued for longer than 24 hours after surgery because it can select for drug-resistant bacterial infections. In human patients, antibiotic-impregnated implants have been used to prevent attachment of bacteria to their surface.22 Minimization of surgery time, careful surgical technique, and use of plate types that are associated with a lower risk of postoperative infection (i.e., non–Slocum-type TPLO plates) may also prevent postsurgical osteomyelitis.12 Metals used in implants should be of the same type (e.g., plate and screws) to minimize the chance of galvanic corrosion. Galvanic corrosion occurs when metals of different electrochemical properties are placed in close proximity in an electrolytic environment. This can predispose to implant loosening, local infection, or sarcoma formation.24 Currently, it is considered acceptable to leave implants in place after healing unless the animal is very young, there is persistent pain associated with the implant, or radiographic evidence of osteomyelitis or osteopenia is present. This is because the process of implant removal itself can be costly and may be followed by osteomyelitis or fracture. However, owners of pets with implants should be advised that although rare, possible long-term complications of implant placement include reactivation of infection in the future or development of malignancy at the implant site.
Osteomyelitis, Discospondylitis, and Infectious Arthritis
Osteomyelitis
Clinical Features
Diagnosis
Diagnostic Imaging
Pathologic Findings
Treatment and Prognosis
Prevention
Osteomyelitis, Discospondylitis, and Infectious Arthritis
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree


