33 Oral joint supplements in the management of osteoarthritis
Introduction
Osteoarthritis (OA) is the single most common cause of lameness in horses (Clegg & Booth 2000) and in one survey, approximately 60% of lameness problems in horses were related to OA (Caron & Genovese 2003). Similarly OA is the most common form of human arthritis, affecting at least 20 million Americans and with its prevalence expected to double over the next two decades (Helmick et al 2008, Lawrence et al 2008). OA involves a complex interaction of biologic and pathologic processes highlighted by eventual degradation of articular cartilage (McIlwraith 1996, 2005). With the US horse population currently estimated to be 7.3 million (AVMA 2007), this means millions of horses currently have this debilitating musculoskeletal condition. Multiple conventional therapies are available for treating OA with the goal of preventing further degradation while restoring function (McIlwraith 2005, Trumble 2005).
Oral joint supplements (OJSs) are a common choice of clients, and have been perceived as a benign treatment for OA in horses (Trumble 2005). The high prevalence of OA in combination with the lack of a definitive cure for OA has probably contributed to the popularity of OJSs among owners, veterinarians, and trainers. These supplements, according to recent market surveys, are the most popular type of nutritional supplements for horses and account for approximately 1/3 (34%) of all equine supplement sales, 1/2 of all pet supplements sold in the US for equine consumption and it is estimated that 49% of all horse owners purchase and administer some form of dietary supplement in their horses (Packaged Facts 2008). In a study of feeding practices in 3-day event horses, the authors found that horses were supplemented with an average of four different oral products daily including electrolytes, plain salt and OJSs (Burk & Williams 2008).
Indications for OJSs
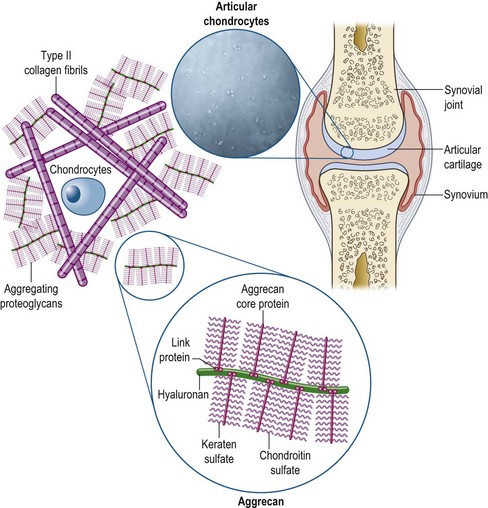
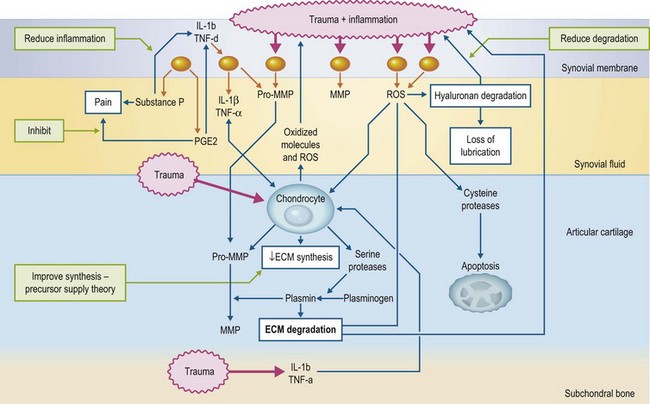
The critical components of articular cartilage are type II collagen fibrils (which provide a structural framework) and extracellular matrix (ECM), which consists of aggrecan molecules and water (Fig. 33.1). The aggrecan molecule consists of aggregations of proteoglycan molecules on a hyaluronan (HA) backbone. The proteoglycan molecule, in turn, consists of a protein backbone with chondroitin sulfate and keratin sulfate side chains. These carry negative charges and due to repulsion as well as some attraction of water provide the compressive resistance to the articular cartilage (McIlwraith 2005). Maintenance of these molecules is critical and some nutraceuticals provide potential building blocks for these molecules. The osteoarthritic process is associated with multiple deleterious mediators from inflamed synovial membrane and trauma as well as being released to initiate a cascade of degradation in the articular cartilage. Interleukin 1 (IL-1) is considered a major cytokine initiating this cascade and this can influence other cells to cause increased release of metalloproteinases, aggrecanase and prostaglandin E2 (PGE2). While this negative process can be influenced by certain nutraceuticals in vitro (Fig. 33.2) there is less certainty about their effects in vivo after undergoing digestion.
In 2005 nutraceutical sales reached more than 1 billion for companion animals, and that number was expected to double in the next 3 years. There are more than 100 equine nutraceutical products currently on the market in the US, and such products are used worldwide. This is a disturbing trend for an industry that for the most part, is unregulated by the FDA or other governing bodies, and has weak in vivo scientific basis (Oke & McIlwraith 2008).
Types of OJSs
Many OJSs have GU and CS as principal components, and may contain additional ingredients including manganese, vitamin C, hyaluronan (hyaluronic acid or HA), polyunsaturated fatty acids (PUFAs), rare earth mineral supplements, unsaponified avocado soy (ASU), cetylmyristoleate, methylsulfonylmethane, and various herbs. With the exception of the latter two (no good equine documentation available), these various products will be discussed below. Box 33.1 discusses the terminology and regulatory issues involved in the use of OSJs.
Box 33.1
Terminology and Regulatory Issues in the Use of Oral Joint Supplements (a US Perspective)
The term nutraceutical was adopted in veterinary medicine from the medical profession and refers to compounds that are neither nutrients nor pharmaceuticals by combining the words “nutrients” (nourishing food or food component) with “pharmaceuticals” (medical drug) (Duren 2005). The nutraceutical category describes a broad list of products sold including nutrients, dietary supplements, functional foods and phytochemicals (including herbs) that are not recognized by the US Food and Drug Administration (FDA) as food or drugs and are intended for the treatment or prevention of disease. The difference between a feed and a nutraceutical is that a nutraceutical is unlikely to have an established nutritive value. Feeds are required to have nutritive value and are accountable, by labeling, for these values. Oral joint supplements fall in between food and drugs and have advantages over either because they are not required to list ingredients or nutrient profiles as required by feeds, and in many cases, intended to treat or prevent disease without first undergoing proper drug approval (Duren 2005).
A supplement was initially defined legally by the Federal Food, Drug and Cosmetic Act (FFDCA), but this was amended in 1994 by the Dietary Supplement Health Education Act (DSHEA). Technically, the DSHEA only covers human products and defines a dietary supplement as a product intended to supplement the diet and contains at least one or more of the following: a vitamin; a mineral; an herb or other botanical; an amino acid; a dietary substance for use to supplement the diet by increasing intakes; or a concentrate, metabolite, constituent, extract or combination of any of the previously mentioned ingredients (McIlwraith 2004). The jurisdiction of veterinary products is primarily the responsibility of the North American Veterinary Nutraceutical Council (NAVNC), which was formed in 1996 to promote and enhance the further quality, safety and long-term effectiveness of nutraceuticals used in veterinary medicine (Trumble 2005). The NAVNC has defined a veterinary nutraceutical as a “non-drug substance that is produced in a purified or extracted form and administered orally to provide agents required for normal bone and body structure and function with the intent of improving the health and well-being of animals” (Booth 2004).
The DSHEA allows manufacturers to make claims with regard to health, structure or function, and nutrient content of a nutraceutical. The Center for Veterinary Medicine allows products to be marketed as nutraceuticals provided they do not claim to treat, cure, or mitigate disease (Trumble 2005); hence, the common practice of advertising of improving joint “health”. The FDA perceives veterinary nutraceuticals as unapproved drugs; however, even though they are not labeled or marketed as drugs. The FDA does not regulate these products unless they become unsafe or have labels that claim a drug use and, therefore, there is no requirement to prove safety or efficacy of a nutraceutical. It is the author’s opinion that there are two principal issues faced with this regulation: (1) The issue is sufficiently low on the FDA radar screen that there is very little attention paid to it, and (2) There is hardly an incentive for a manufacturer of a nutraceutical to prove efficacy, as such research costs money and a negative result could hurt sales (McIlwraith 2004).
Manufacturers do not have to register themselves or their supplements with the FDA. In general a manufacturer has to comply with the FDA Ingredient Recognition Program, which entails applying for complete ingredient definitions as described by not-for-profit organization of state and federal feed regulators, the Association of American Feed Control Officials (AAFCO) (McIlwraith 2004). There are no requirements of Good Manufacturing Practices (GMPs) for manufactures to guarantee high-quality and batch-to-batch consistency (Oke & McIlwraith 2008) and because there is no post-production monitoring of veterinary nutritional supplements, a myriad of poor quality supplements are available (Oke et al 2006).
Glucosamine and/or chondroitin sulfate
Mechanisms of action and in vitro studies
In vitro studies have investigated the effects of glucosamine and CS, individually or in combination, but of course, differ greatly in the source of products, as well as dosages used, in vitro study conditions and responses measured (Dechant & Baxter 2007). Glucosamine and/or CS are thought to counteract cartilage degradation (Sandy et al 1998, Fenton et al 2000, 2002, Grande et al 2000, Lippiello et al 2000, 2002, Nerucci et al 2000, Orth et al 2002, Ilic et al 2003, Mello et al 2004, Dechant et al 2005, Neil et al 2005b) by inhibiting degradative enzymes such as collagenase and aggrecanase (Wright 2001, Lippiello et al 2002, Orth et al 2002, Mello et al 2004, Chan et al 2005), and intermediary mediators, such as nitric oxide, prostaglandin E2 and nuclear factor kappa B (Bassleer et al 1998a, Fenton et al 2000, 2002, Orth et al 2002, Byron et al 2003, Dodge et al 2005, Largo et al 2003, Mello et al 2004, Nakamura et al 2004, Schlueter et al 2004, Neil et al 2005b). Synthesis of ECM components is thought to be stimulated in the presence of GU and/or CS (Bassleer et al 1998a,b, Nerucci et al 2000, Noyszewski et al 2001, Lippiello 2003, Dechant et al 2005) by provision of substrate, which may be deficient through dilution or hypermetabolic states (Lippiello 2003); upregulation of gene expression (Grande et al 2000, Dodge & Jimenez 2003); stimulation of cellular receptors and cellular signaling mechanisms, such as CD44 involved in positive feedback (Bassleer et al 1998a, Esford et al 1998, Platt 2001); and inhibition of negative intermediary messengers, such as nuclear factor kappa B, nitric oxide and prostaglandin E2 (PGE2) (Bassleer et al 1998a, Fenton et al 2000, 2002, Orth et al 2002, Largo et al 2003, Mello et al 2004, Nakamura et al 2004, Schlueter et al 2004).
The Precursor Supply Theory is the most popular explanation regarding the apparent beneficial effects of GU in OA (Oke & Weese 2006). In this theory it is proposed that GU supplies excess basic building blocks for the synthesis of cartilage glycosaminoglycans (GAG) (Fenton et al 2000, Laverty et al 2005, Kelly 1998) and/or bypasses rate-limiting steps in GAG synthesis (Fenton et al 2000, Trumble 2005). In addition, these structure-modifying agents appear to counteract inflammation primarily through their inhibition of intermediate messengers, such as nuclear factor kappa B, nitric oxide and PGE2 (Bassleer et al 1998a, Fenton et al 2000, 2002, Orth et al 2002, Largo et al 2003, Mello et al 2004, Nakamura et al 2004, Schlueter et al 2004, Neil et al 2005b), that mediate inflammatory responses, in addition to their previously described antianabolic and procatabolic effects. However, these structure-modifying agents have not been found to directly inhibit cyclooxygenase (COX) enzymes, in contrast to many antiarthritic medications (Seaver & Smith 2004). CS has also been shown to affect cell-based inflammatory events by inhibiting chemotaxis, reducing phagocytosis and lysozyme release, and protecting cell membranes from free radical injury (Ronca et al 1998).
It has been pointed out by Dechant and Baxter (2007) that the dosages used to determine the effects of GU and CS on cartilage metabolism in in vitro studies have varied from physiological (µg/ml) to pharmacological (mg/ml) concentrations. Depending on the study, beneficial effects have been reported for dosages as low as 10 µg/ml to as high as 25 mg/ml. Some studies have compared combination treatments to glucosamine or CS alone (Grande et al 2000, Lippiello et al 2000, Orth et al 2002, Dechant et al 2005). Combinations of GU and CS were considered to be the most effective in these studies (Grande et al 2000, Lippiello et al 2000, Orth et al 2002, Dechant et al 2005) and although synergy was suggested by some authors (Lippiello et al 2000), the effect tended to be additive and not synergistic (Dechant & Baxter 2007).
The effects of varying doses of GU and CS alone and in combination on cartilage metabolism in normal and recombinant IL-1α conditioned equine articular cartilage has been evaluated in the author’s laboratory using equine cartilage explants (Dechant et al 2005). Articular cartilage explants were allocated randomly to treatment with four doses of GU, CS, or GU + CS in the absence of IL-1 (normal explants) for treatment with four dosages of GU, CS, or GU + CS in the presence of 40 mg/ml recombinant IL-1α (Gibco-Light Technologies, Grand Island, NY, USA) (IL-1 conditioned explants). The patented joint supplements used were SCHG49GU and TRH122 low molecular weight sodium (Nutramax Laboratories, Edgewood, MD, USA). The treatment groups investigated for both normal and IL-1 conditioned explants were: treatment without GU or CS; four GU concentrations, 12.5, 25, 125 and 250 µg/ml; four CS concentrations, 12.5, 25, 125 and 250 µg/ml; and four GU + CS concentrations 12.5, 25, 125 and 250 µg/ml each of GU and CS (in a 1 : 1 ratio by concentration). There was no significant negative effect of GU, CS, or GU + CS on normal cartilage explant metabolism. In normal (no IL-1) explants, the most substantial effects observed with the GU, CS and GU + CS treatment were in reducing GAG degradation, without evidence for an advantage of GU + CS compared to GU or CS alone. On the other hand, the highest dosage of GU + CS was more effective than all other treatments in reducing GAG degradation in IL-1 conditioned explants. The ability of GU and CS to protect against cartilage matrix degradation in osteoarthritic and stimulated chondrocytes in cartilage explants have been observed in other in vitro studies (Sandy et al 1998, Fenton et al 2000, 2002, Grande et al 2000, Lippiello 2000, 2002, Nerucci et al 2000, Orth et al 2002, Byron et al 2003). It was also noted that the higher doses of test ingredients (125 and 250 µg/ml) tended to be more effective than the lower dosages (Dechant et al 2005) and these dosage ranges were within the ranges of other in vitro studies. It has been pointed out that high dosages of GU such as 6.5 and 25 mg/ml have been shown to have detrimental effects on cartilage metabolism and chondrocyte viability in studies using bovine articular cartilage explants (De Mattei et al 2002).
Key Points
• Purported mechanisms explaining the role for glucosamine (GU) in osteoarthritis are: (1) provision of substrate for synthesis of cartilage glycosaminoglycans (precursor supply theory); and (2) mediation of inflammatory responses
• Chondroitin sulfate (GS) is thought to counteract cartilage inflammatory responses
• There is in vitro evidence that GU and CS protect against cartilage matrix degradation