Chapter 20 Ophthalmology of Exotic Pets
Ocular anatomy of the vertebrate has been remarkably conserved throughout evolution. Structures universally present in these eyes are an outer fibrous tunic (cornea and sclera), middle vascular tunic or uvea (iris, ciliary body, choroid), inner neural tunic (retina), and internal optical media (aqueous humor, lens, vitreous). This basic pattern, particularly as it applies to mammals, is outlined in Chapter 1. Familiarity with general ocular anatomy and physiology is crucial to understanding the clinical signs and pathogenesis of ophthalmic disease. The objective of this chapter is to discuss differences in ocular anatomy and provide the general practitioner with information pertaining to the ophthalmic examination and diagnostic testing, common ophthalmic diseases, and their treatments in exotic species.
OPHTHALMIC EXAMINATION AND DIAGNOSTIC TESTING
The techniques used in the ophthalmic examination are no different whether one is examining an exotic species or a dog or cat. A guide to these techniques is outlined in Chapter 5. However, evaluation of the tear film, intraocular pressure (IOP), and fundus may be more difficult in exotic animals owing to their smaller globes.
The most common method of evaluating the tear film is through the quantification of aqueous tear production with a Schirmer tear test (see Chapter 9). The strips used for the test are 5 mm wide, making their use difficult in eyes in which palpebral fissure length is less than 1cm. Historically, these strips have been cut lengthwise to decrease their width, allowing easier placement. However, because this practice is not standardized, there is large variability between results, making interpretation of values difficult.
A phenol red thread test is more appropriate for the smaller eyes of exotic animals. The test is performed similarly to the Schirmer tear test. The thread is placed in the lateral canthus for 15 seconds, and the millimeters of wetting are measured. Normal values for the Schirmer tear test and phenol red thread test for a number of species are shown in Table 20-1. Qualitative tear film deficiencies are currently diagnosed in canine and feline ophthalmology through the use of the tear film break-up time. This technique is not yet in common usage with exotic species.
Table 20-1 Normal Values for Tear Quantification Tests in Exotic Species
| SPECIES | SCHIRMER TEAR TEST RESULT | PHENOL RED THREAD TEST RESULT |
|---|---|---|
| Rabbit | 5 ± 3 mm/min | 24 ± 4 mm in 15 sec |
| Rat | 8 ± 3 mm in 5 min | 14 ± 3 mm in 2 min |
| Mice | 3 ± 0.2 mm in 2 min* | 3 ± 1 mm/min |
| Birds (Psittaciformes) | 5 ± 3 mm/min | 22 ± 4 mm in 15 sec |
* Modified Schirmer tear test strip.
IOP evaluation, or tonometry, can readily be performed in conscious exotic animals. Two types of tonometers are most frequently used to evaluate IOP, an applanation tonometer (Tono-Pen) and rebound tonometer (TonoVet). Published IOP values for a number of exotic animal species are shown in Table 20-2.
Table 20-2 Normal Intraocular Pressure Values for Exotic Species
| SPECIES | TONOMETRY VALUE (mm Hg) |
|---|---|
| Rabbit | 13 ± 6 |
| Ferret | 23 ± 5 |
| Mice | 15 ± 5 |
| Rat | 15 ± 5 |
| Birds (raptors) | 20 ± 4 |
| Owls | 11 ± 4 |
The small globe (short axial length) of many exotic animals increases the difficulty of critically examining the fundus. However, performing indirect ophthalmoscopy should be a standard part of the ophthalmic examination and is facilitated through pharmacologic mydriasis and use of a highly refractive indirect lens. Lenses of 30, 40, or 60 D are particularly useful. Pupillary dilation in mammals is achieved with 1% tropicamide, usually within 20 minutes and persists for 4 to 8 hours. The pupils of birds and reptiles will not dilate with anticholinergic agents owing to the presence of striated muscle fibers in both the pupillary sphincter and dilator muscles. Achieving mydriasis in these species is more difficult and may require intracameral infusion of curariform neuromuscular blocking agents. More recently, topical application of vecuronium bromide (1 drop twice at a 15-minute interval) with or without the addition of phenylephrine and atropine has been demonstrated to cause consistent mydriasis lasting 1.5 to 4 hours in birds. Other curariform neuromuscular blocking agents used topically have been associated with severe side effects, including paralysis of muscles outside the eye, especially those of respiration. Caution should always be exercised in the use of these agents topically or intracamerally. Their use has not yet been evaluated in reptiles. Mydriasis may also be achieved with general anesthesia, although this method has its own inherent risks and complications.
Other diagnostic tests, including fluorescein and rose bengal staining, exfoliative cytology, and microbial culture, are completed as they are in the more common domestic species; they are described in detail in Chapter 5.
RABBITS
Orbital Disease
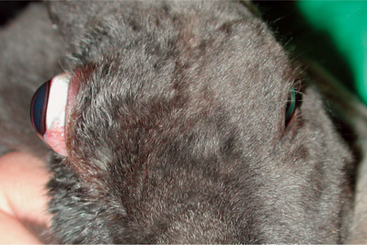
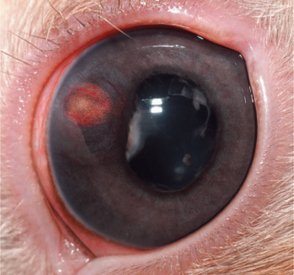
Exophthalmos is the most prevalent clinical sign associated with orbital disease. In rabbits, retrobulbar abscesses are commonly the cause of exophthalmos (Figure 20-1). Pasteurella multocida has frequently been implicated as the cause for these abscesses, although confirmation through culture is rarely attained. Regardless of the specific pathogen responsible, it likely gains access to the orbit either through infected molar tooth roots or from hematogenous spread. Diagnosis is made through clinical signs and ocular ultrasonography. Successful treatment is difficult. The abscess cannot be drained through the oral cavity because of the rich vascular plexus lining the rabbit orbit as well as the extremely caseous nature of the contents. Many cases require exenteration and long-term medical therapy with a broad-spectrum antibiotic. True Pasteurella-induced abscesses may respond favorably to parenteral penicillin therapy at a dose of 60,000IU/kg q24h for several weeks. However, the use of penicillins in rabbits warrants extreme caution because they can cause anaphylaxis or fatal enterocolitis due to alteration of the normal gastrointestinal flora. Even with aggressive surgical and medical therapy, many of these abscesses recur and at times necessitate euthanasia. Less common causes of exophthalmos in rabbits include parasitic cysts, orbital neoplasia, and metastatic thymoma.
Adnexal Disease
Adnexal disease is uncommon in rabbits, with entropion being the most commonly observed abnormality. Entropion may involve the upper and/or lower eyelids and may be primary or secondary. Primary (congenital) entropion has been described in the New Zealand white and French lop breeds. These breed predispositions suggest an inherited basis, but a mode of inheritance has not been determined. The lower eyelids are most commonly affected in New Zealand white rabbits, whereas the upper eyelids are affected in French lops. Clinical signs associated with primary entropion include epiphora, blepharospasm, conjunctival hyperemia, and, sometimes, ulcerative keratitis (Figure 20-2). Application of a topical anesthetic such as 1% proparacaine will facilitate ophthalmic examination by alleviating surface ocular pain. It will also remove any spastic component to the entropion, allowing appropriate surgical planning. Correction of entropion with a modified Hotz-Celsus procedure allows restoration of normal anatomic conformation, thereby preventing further ocular irritation.
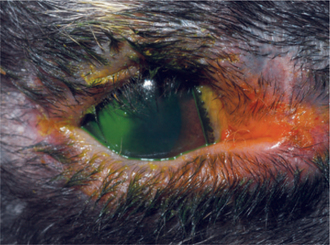
Entropion may occur from cicatrix formation secondary to chronic blepharitis or blepharoconjunctivitis. Before any attempt at surgical correction, the underlying cause must be identified and treated. Otherwise entropion will likely recur. Cicatricial entropion is corrected using a Y-to-V blepharoplasty, with care taken to completely excise the offending fibrotic tissue during the procedure. Infectious blepharitis may result from exposure to Treponema cuniculi, or rabbit syphilis (Figure 20-3). T. cuniculi is a spirochete bacillus transmitted by infected dams. The most commonly affected areas are the vulva, prepuce, lips, nares, and anus. Clinical signs include thickening, crusting, and, possibly, ulceration of the eyelid. Diagnosis is confirmed through identification of spirochetes in biopsy or skin scraping samples. Darkfield microscopy enhances visualization of the organism. Three doses of parenteral benzathine penicillin G or procaine penicillin G at a dose of 42,000IU/kg at 7-day intervals is the treatment of choice. Careful monitoring of the patient for potentially fatal enterocolitis is necessary. Blepharitis may also be caused by self-induced trauma or maceration due to ocular discharge secondary to dacryocystitis, conjunctivitis, or keratitis (discussed later).
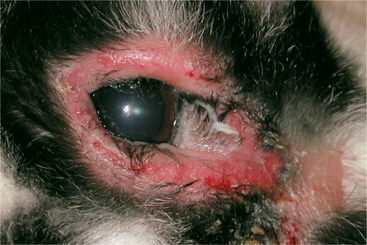
Figure 20-3 Marked blepharitis and severe mucopurulent ocular discharge in a rabbit with Treponema cuniculi.
Conjunctival Disease
Viral causes of ophthalmic disease are relatively uncommon in domestic rabbits in the United States. Myxoma virus is a member of the Poxviridae family and is endemic in the western United States (especially among brush rabbits in California), Europe, and Australia. The virus is transmitted by an arthropod vector, including both mosquitoes and fleas. Clinical signs are blepharoconjunctivitis with a thick mucopurulent discharge, blepharedema, and edema and nodule formation on the ears, head, body, and limbs. Myxoma virus was formally thought to have 100% mortality, but more recent data suggest that the prognosis varies according to the strain of the virus and species/breed of rabbit. Diagnosis is obtained from clinical signs, gross pathology findings, and polymerase chain reaction (PCR) analysis of tissue extracts. Treatment is supportive only.
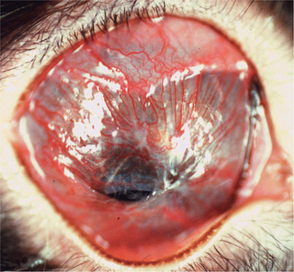
Formation of a conjunctival membrane over the corneal surface is a condition unique to rabbits. This condition has been labeled pseudopterygium, corneal occlusion syndrome, conjunctival centripetalization, and epicorneal membrane (Figure 20-4). Progressive ingrowth of the bulbar conjunctiva occurs symmetrically and centripetally. The conjunctiva does not adhere to the corneal surface, separating this condition from true pterygium described in humans. The disease may be unilateral or bilateral. The cause has not been determined, although a collagen dysplasia has been proposed. Surgical correction is completed through a modified Arlt procedure. The conjunctival tissue is partially trimmed by sharp dissection, divided in half along the horizontal axis, and the leading edge is sutured to the conjunctival fornix. Because of the likelihood that this disease is immune mediated, application of cyclosporine, mitomycin C, or a steroid may decrease recurrence. However, topical administration of these agents may result in systemic immunosuppression and therefore they must be used with caution.
Corneal Disease
The large, prominent globes and decreased blink rate of rabbits may predispose them to corneal disease. Corneal ulceration is common; it may be associated with trauma, exposure after anesthesia, distichiasis, entropion, and trichiasis and may occur secondary to blepharoconjunctivitis or dacryocystitis. Thorough examination will aid in differentiating the underlying cause. If no conformational abnormality is noted, trauma can be assumed. Diagnosis is facilitated by the use of fluorescein stain as in other species. Uncomplicated defects are treated symptomatically with a broad-spectrum topical antibiotic, such as chloramphenicol, several times daily. Ulcers that do not heal, have significant stromal loss, or are infected are deemed “complicated” and require more intensive therapy. Nonhealing or indolent corneal ulcers are common and should be treated like those in dogs. Aggressive corneal débridement with a cotton-tipped applicator after topical anesthesia is recommended. A grid or multiple superficial punctate keratotomy may also be necessary to promote healing. Cytology and culture and sensitivity testing of infected corneal ulcers are recommended so that appropriate antibiotic therapy can be initiated. When there is significant stromal loss or a descemetocele is present, referral to an ophthalmologist is warranted. Placement of a conjunctival pedicle graft is the treatment of choice, although this procedure is more difficult in rabbits owing to their notably thin cornea (0.36mm) compared with dogs (0.56mm). After surgery, antimicrobial therapy is initiated on the basis of cytology and culture results. Reflex uveitis secondary to keratitis likely occurs in rabbits as it does in dogs, and application of atropine may help alleviate ciliary spasm associated with uveitis through cycloplegia. However, the presence of atropinase in the rabbit uvea may prevent its effectiveness. Because atropinase is not universally present and not all rabbits are immune to atropine’s effects, its application is recommended. During treatment of corneal disease in rabbits, ophthalmic solutions or suspensions are preferred to ointments because of their normal grooming behaviors.
Cataract
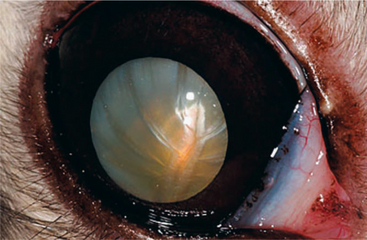
Cataracts may occur spontaneously or may be secondary to uveitis in rabbits. Some spontaneous cataracts (Figure 20-5) are suspected to be inherited in nature, although no genetic evidence is available because of the low incidence. Evaluation of laboratory New Zealand white rabbits revealed a 4% prevalence of spontaneous cataracts with no difference between males and females. Treatment is necessary only if secondary lens-induced uveitis or visual deficits are present. Removal of the lens via phacoemulsification is the treatment of choice. The procedure is similar to that performed in dogs, although the surgeon must take care upon entering the anterior chamber, because it is shallow and iris prolapse through the incision is more likely. Additionally, meticulous irrigation/aspiration of the peripheral lens cortex is necessary owing to the greater likelihood of lens fiber regrowth.
Intralenticular organisms have been identified in approximately 75% of rabbits with cataract formation with secondary phacoclastic uveitis. These organisms were 1 to 6mm in size and consistent with the obligate intracellular protozoa, Encephalitozoon cuniculi. Rabbits are infected with E. cuniculi after ingestion of food contaminated with urine. Nonocular signs include neurologic disease (head tilt, torticollis, seizures, ataxia, paralysis) and renal disease. Lens infection occurs in utero owing to vertical transmission from an infected dam. Dwarf and young rabbits are predisposed. Clinical signs are usually unilateral and include spontaneous lens capsule rupture with resultant phacoclastic uveitis (including aqueous flare, hypopyon, and hypotony), iridal granuloma formation, and cataract formation (Figures 20-6 and 20-7). Diagnosis is based on clinical signs along with supportive results from serology (indirect fluorescent antibody test, enzyme-linked immunosorbent assay) or PCR analysis of lens tissue. Serology is quite sensitive, as rabbits mount a humoral response within 2 weeks, and in one study 100% of rabbits with ocular signs were seropositive.
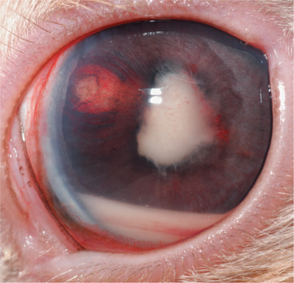
Figure 20-7 Same rabbit as in Figure 20-6, after 2 weeks without therapy. Phacoclastic uveitis is present as evidenced by lens capsule rupture, cataract formation, hyphema, and hypopyon.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree