Use of the Liver Activity Index and Other Metabolic Variables in the Assessment of Metabolic Health in Dairy Herds
Giuseppe Bertoni∗ and Erminio Trevisi, PhD, Institute of Zootechnic, Faculty of Agriculture, Università Cattolica del Sacro Cuore, Via Emilia Parmense, 84, Piacenza 29122, Italy. E-mail address: giuseppe.bertoni@unicatt.it ∗Corresponding author.
Keywords
Metabolic profile
Inflammation
Sampling standardization
Liver functionality
Transition period
Dairy cows
Introduction
Most metabolic diseases (milk fever, ketosis, retained placenta, and displacement of abomasum) of dairy cows occur within the first 2 weeks of lactation.1 In the same period the majority of infectious diseases (eg, mastitis, metritis) also occur. Furthermore, some other metabolic diseases that manifest clinically later in the lactation (eg, laminitis) can be traced back to metabolic insults that occurred during early lactation (ie, rumen acidosis as suggested by Nocek2). One of the reasons, particularly of the higher frequency of infectious disease, is the immune depression typical of this period.3 This depression is often attributed to energy deficiency, and is related to higher serum concentrations of nonesterified fatty acids (NEFA) and β-hydroxybutyric acid (BHBA).4 There is therefore a close relationship between metabolic and infectious diseases; both of these types of disease may lead to inflammation, which can be responsible for lower milk production and impaired fertility.5,6 Poor reproductive performance, like poor health and production, may also be traced back to problems occurring in the transition stage.
The ever increasing milk yields of modern dairy cows are generally considered as an important risk factor in the development of metabolic disease. However, this is only partly true because nutritional (inadequate feed intake) and environmental stressors are also of relevant importance.7 This issue has been recently discussed with respect to the apparent antagonistic association between milk production and fertility.8 The investigators suggest that in the case of multifactorial associations, such as those between milk yield and fertility, it is essential to understand the complexities of the variables affecting fertility (i.e. the relationships between milk production, nutrition, and metabolic diseases). Then, it is of paramount importance, particularly for high-yield cows, to exercise great care in feeding9,10 and management so as to minimize multiple stressors.11
Consequently, there is intense interest in nutrition and management during the transition phase4,12 and perhaps some time before (eg, at the end of previous lactation to avoid overfattening). Attention to feeding details and adherence to recognized nutritional guidelines13,14 is the first step in managing metabolic disease. However, additional strategic tools may be useful in reducing the overwhelming risks during the transition period, one of which is appropriate application of blood analysis. There are 2 main aims in such analyses:
• Herd-level evaluation to assess overall feeding and management adequacy in dry and early lactation periods
• Cow-level evaluation to assess the status of individual animals relative to clinical or subclinical disease, the objective being to monitor herd incidences of disease and to provide an opportunity for early treatment of prophylaxis
Herd-level metabolic evaluation
Historically the use of blood analysis to evaluate the metabolic health of cows can be traced back to Payne and colleagues15 and their metabolic profile, which can be considered a presymptomatic diagnostic tool to prevent metabolic disorders and diseases in dairy farms. This approach was later almost abandoned, owing to cost and insensitivity relative to nutritional inadequacies.16 Today it seems counterintuitive to view metabolic profiles as a means of ration evaluation because precise means of ration formulation and evaluation are otherwise available.17 By contrast, there is opportunity to use blood analysis as the last step in an Integrated Diagnostic System (IDS) to solve nutrition-management problems at the farm level. The first step is the collection of available data concerning the herd, namely feeding, management, performance, environment, body condition score (BCS), disease frequency, or any other problem,18 thus avoiding new analysis. This approach obviously implies that blood profiling (second step) is recommended for the diagnosis and evaluation of problems not identified in the first step. Blood profiling would be used only when other more simple and less expensive diagnostic tools have failed. To properly interpret metabolic profiles, Bertoni and colleagues18 suggest well-standardized techniques for sample collection and analysis, as well as an appropriate approach to the evaluation of blood changes based on their biochemical meaning and on specific reference values. For this purpose, namely to evaluate the metabolic status in cows without clear clinical signs of disease, the variation in blood analyte concentrations associated with subclinical abnormalities will not be as extreme as in the case of clinical disease. Thus, meticulous attention should be given to the avoidance of extraneous causes of variation in blood variables in the use of reference ranges specifically established for this purpose.
Minimizing Extraneous Sources of Variation in Blood Analyte Concentrations
Animal selection and sampling
Sources of variation other than those expected in response to metabolic or nutritional challenges, that is, the response variables of interest, may be minimized by proper animal selection, proper timing of sample collection, and proper sample handling after collection. Animals showing signs of clinical disease should not be selected when sampling for herd-level evaluation. Clinical disease frequently causes secondary changes in blood variables that can interfere with herd-level interpretation. Furthermore, timing of sampling relative to feeding is important, as there is prandial variation among several metabolites of interest including glucose, NEFA, and BHBA as well as among some hormones such as insulin, thyroid hormones, and growth hormone.19,20 Prandial effects may be reduced when totally mixed rations (TMR) diets are available continually throughout the day,21 but even in these types of feed delivery systems it is most desirable to sample just before daily delivery of fresh feed. During restraint and sample collection animals should remain as calm as possible to avoid stress-related changes in blood variables. For some variables, sampling site (jugular, mammary, or coccygeal vein) is also a potential cause of extraneous variation. Selection of sampling site may also affect the risk of sample hemolysis (higher in tail samples), which should be avoided.22 Box 1 lists specific instructions for animal selection and sampling.
Sample analysis: Analytical techniques are often beyond the control of the clinician, but it is advisable to understand the control procedures used by the laboratory. The use of bovine-origin control samples is recommended, as is the use of 1 or 2 commercial standards for the calibration of equipment.23
Interpretation
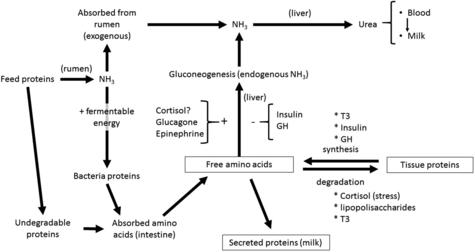
• Blood urea is not an index of rumen ammonia concentration only, as is often supposed.24,25 Amino acid oxidation is also an important factor.26,27 Thus, a high intake of any protein source tends to increase blood urea concentration (Fig. 1).
• The sudden and marked decrease in serum zinc concentration, and partly of serum calcium concentration, is generally due to inflammatory phenomena,28,29 almost never due to deficiency.
• Reduced serum concentrations of albumin, cholesterol, and vitamin A can at least partly be a consequence of a reduced liver synthesis of usual proteins; lower lipoprotein concentrations result in lower serum cholesterol, and lower serum retinol-binding protein concentrations result in lower vitamin A. This situation can occur during an acute-phase reaction that diverts the activity of the liver to the synthesis of other proteins such as haptoglobin, fibrinogen, C-reactive protein, ceruloplasmin, or metallothionein, instead of the aforementioned more normal hepatic protein products.16,30
• Glucose, NEFA, and BHBA are useful as energy indicators, but mainly at the end of pregnancy and in the first weeks of lactation, or in the case of great and prolonged energy deficiencies, the latter being an infrequent occurrence in dairy cows.
• Among minerals, only P (inorganic), Mg, Se, and I can be monitored in blood to establish their dietary availability.
The use of reference values specific to the metabolic profile application is a critical point. In fact, given the aims, which are to detect herd-level deviation of blood values in clinically normal cows, one cannot use the usual clinical biochemistry reference values, which are designed to identify animals with clinical illness. For many years31 our Institute has maintained that reference values for the interpretation of dairy herd metabolic profiles must be obtained with proper trials, performed on clinically healthy animals, correctly fed and managed, and also (we can add today) without important inflammatory events. The same concepts have been substantially expressed by the International Federation of Clinical Chemistry (IFCC) for humans, through many contributions to the theory of reference values (1987–1991). In particular, IFCC rules suggest that “reference values from an individual or a group of individuals are only meaningful when the individual(s) and method of production of values are adequately described.” Consequently, to obtain these appropriate reference values for herd-level metabolic profile interpretation, a population of reference herds must be established. These herds should be well managed, appropriately fed, and with a low frequency of clinical diseases. Furthermore, the selection of animals, method of sample collection and handling, and methods of sample analysis should all be standardized, as already discussed. Using these criteria, the reference values obtained for dairy cows are shown in Table 1.32 These values may vary depending on the physiologic stage (dry vs fresh) or age (primiparous vs multiparous) of animals.
Table 1
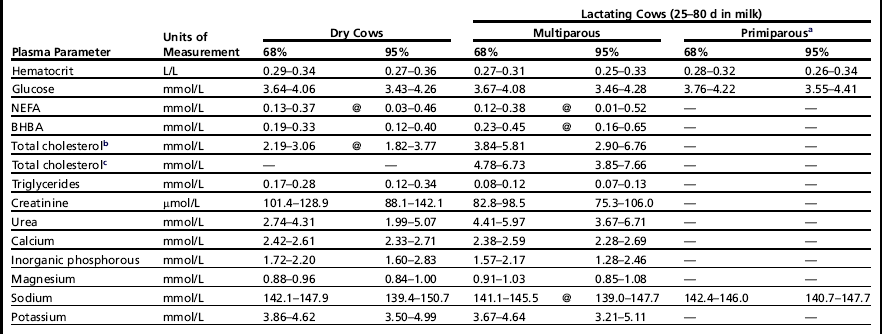
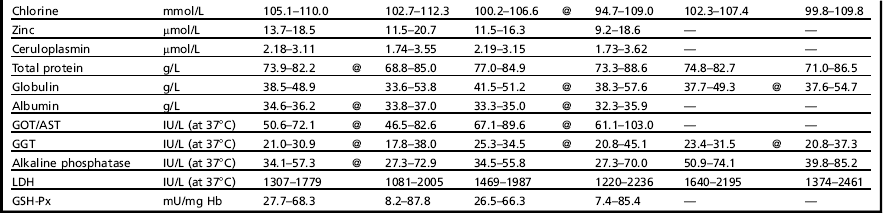
aFor the primiparous cows, only shown are the reference values statistically different in comparison with multiparous cows.
bDiets without fat supplementation.
cDiets with fat supplementation.
Data from Bertoni G, Calamari L, Trevisi E. Nuovi criteri per l’individuazione dei valori di riferimento di taluni parametri ematici in bovine da latte. La Selezione Veterinaria 2000;Suppl:S261–8.
Nevertheless, the metabolic profile (blood analysis) could be included in an IDS aimed to solve any kind of farm problems.18 The first step is to create a precise clinical evaluation of the farm (management conditions for animals, health problems, feeds and diets, animal characteristics [eg, BCS, cleanliness, teat score], digestive function [rumination, fecal characteristics], milk yield and quality [eg, fat, protein, somatic cells number], animal behavior, and so forth. The metabolic profile, as a second step, could be a useful complement for the better understanding of more difficult situations such as subclinical inflammations (see the following sections of this article and the article by Sordillo and colleagues elsewhere in this issue), which can be the consequence of nutritional errors (eg, excess of energy, deficiency of specific nutrients), digestive disorders, and infections. Inflammation, as may be present at a low grade, represents a metabolic disease risk per se and may interfere with nutrition balance.
Special Laboratory Procedures for Evaluation of Health in Transition Cows
In dairy cows, the first phase of lactation is characterized by a high incidence of diseases such as mastitis and endometritis,33 and also metabolic disorders, particularly in high-producing dairy cows.34 These metabolic disorders can have their root cause in the last weeks of pregnancy. The several diseases are related to each other, and the existence of one disease can increase the risk for others and vice versa. This relationship explains the typical syndromes of multiple diseases occurring during the peripartum period. It also illustrates the challenge in properly separating the root sources of each problem, which is essential in diagnosing the fundamental causes of disease. This complexity leads to the observation of Chapinal and colleagues35 that “maintaining health and productivity in the transition period is one of the most difficult challenges that dairy herds face.” Moreover, the importance of meeting this challenge becomes even more critical because the occurrence of metabolic diseases in early lactation is strongly and negatively associated with subsequent fertility.6,36 Again this suggests the diagnostic utility of metabolic indicators that could be “… associated with herd-level incidence of retained placenta, metritis and displaced abomasum, milk production, and probability of pregnancy at the first artificial insemination.”35
Blood variables as metabolic indicators could be useful as monitors of metabolic health, but the situation is very complex because the causes and effects of transition-cow diseases occur within the framework of the tremendous physiologic changes (endocrine and metabolic) characteristic of the transition period. These physiologic changes are part of some complex mechanisms needed, first to prepare the cow for parturition and lactogenesis, and then to achieve the homeorhetic changes37 required to sustain milk synthesis despite negative nutrient balance.
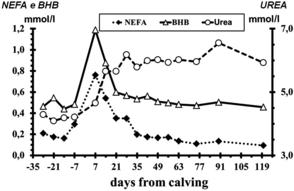
In this context, the better known blood changes concern those of glucose, NEFA, BHBA, and urea (Fig. 2),38 indices of energy and protein balance; nevertheless, the recent articles of Chapinal and colleagues35 and Lomander and colleagues36 have concluded that, despite their usefulness, the suggested indices (NEFA, BHBA, and Ca) need further research to understand how the thresholds can be applied or which is the best protocol for blood sampling (see also the article of Opsina and colleagues elsewhere in this issue for a discussion of use and interpretation of serum NEFA and BHBA concentrations in transition cows). A potential confounding factor in the interpretation of serum NEFA, BHBA, and other metabolite concentrations is the presence of inflammation and inflammatory mediator substances. Whereas metabolic events can influence inflammation (see the article by Sordillo and colleagues elsewhere in this issue), inflammatory variables are only partly associated with metabolism. Inflammatory variables are, however, strongly associated with health during the transition period.5,39,40 Recent observations of Van Knegsel and colleagues41 concerning the concentrations of natural antibodies in blood further support the association of metabolic and inflammatory conditions in transition dairy cows. This aspect is of great interest for several reasons. Inflammatory conditions, not only clinical but also subclinical, occur very often in the transition period and can result in lower milk yield and fertility.5 Furthermore, inflammation may contribute to reduced feed intake (dry matter intake [DMI]) and efficiency of energy use,42,43 all of which could contribute to challenges to metabolic adaptation and health.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree