CHAPTER 14 Development of the gastro-pulmonary system
With the cranio-caudal and lateral foldings of the trilaminar embryonic disc, the primitive yolk sac, lined by the endoderm dorsally and by the hypoblast laterally and ventrally, is divided into an intra-embryonic tube lined by endoderm and an extra-embryonic yolk sac lined by hypoblast (Fig. 14-1). These two compartments initially communicate through a wide opening at the developing umbilicus; later, this communication becomes narrowed to a thin vitelline duct that persists for as long as the yolk sac is present (see Chapter 9). The intra-embryonic tube, lined by endoderm and covered by splanchnic mesoderm, is referred to as the primitive gut.
The primitive gut is divided into three parts. The cranial portion, the foregut, is blind, closed cranially by the oro-pharyngeal membrane which is lined by endoderm on the inside and covered by ectoderm on the outside (see Chapter 7). The caudal portion, the hindgut, is also blind, being closed by the cloacal membrane, similarly lined by endoderm and covered by ectoderm. A connecting portion, the midgut, opens into the yolk sac. An ectodermal depression at the location of the oro-pharyngeal membrane, the stomodeum, later develops into the oral cavity, while a similar depression at the site of the cloacal membrane, the proctodeum, develops into the anus and the opening of the uro-genital system.
The primitive gut is suspended from the dorsal and ventral body walls by mesenteries consisting of double layers of peritoneum developed from the mesoderm. The dorsal mesentery extends from the caudal end of the developing oesophagus to the cloacal region of the hindgut and gives rise to the greater omentum and the mesentery of the gut. The ventral mesentery develops from the septum transversum, and becomes restricted to the caudal portion of the foregut, i.e. the caudal oesophagus, the stomach and the initial portion of the duodenum. The developing liver grows into the ventral mesenterium and the septum transversum, and the former develops into the lesser omentum. One part of the ventral mesenterium, the falciform ligament, carries the left umbilical vein from the umbilicus to the liver (see Chapter 12).
The primitive gut gives rise to a number of endodermal derivatives (Fig. 14-2). From the foregut come derivatives of the pharyngeal arches, pouches and clefts, the respiratory system, the oesophagus, the stomach as well as the liver and pancreas. The midgut forms most of the intestine, and the hindgut gives rise to caudal parts of the intestine and also the allantois (see Chapter 9).
Finally, it should be mentioned that the gastrointestinal system develops an extensive nerve supply referred to as the enteric nervous system; numerous neural crest cells invade the investments of the gastrointestinal system and form ganglia in the submucosal and myenteric plexuses (see Chapter 10).
THE ORAL AND NASAL CAVITY AND PALATE
The primary oral cavity develops from the stomodeum as the oro-pharyngeal membrane regresses to connect the endodermally-lined primitive gut to the ectodermally-lined stomodeum (Fig. 14-2). The demarcation between the ectoderm- and endoderm-derived portions of the definitive oral and nasal cavities cannot be distinguished.
The primary nasal and oral cavities
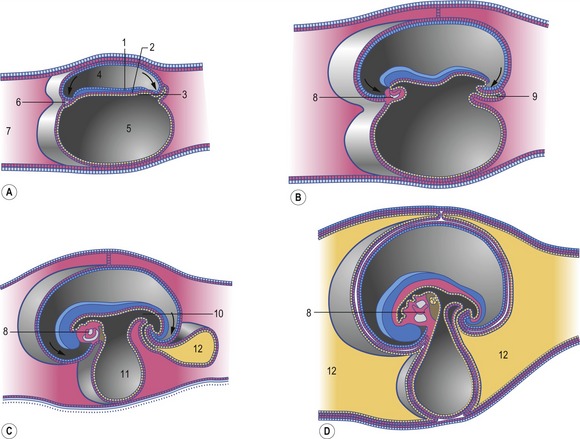
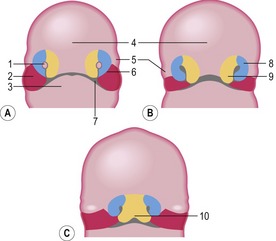
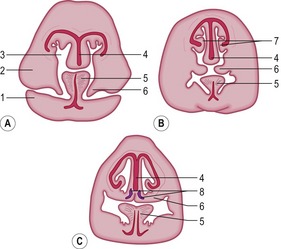
The first recognizable facial structures are the fronto-nasal prominence and the paired maxillary and mandibular prominences. These develop dorsally, laterally and ventrally, respectively, to the entrance to the stomodeum (Fig. 14-3). The maxillary and mandibular prominences are derived from the first pharyngeal arch (see later). The ectoderm of the fronto-nasal prominence differentiates into the paired nasal (olfactory) and lens placodes. The latter develop into the lenses of the eyes (Chapter 11). Medial and lateral nasal prominences subsequently develop at each side of the nasal placodes. Gradually, the nasal placode invaginates to form the nasal pit which develops into the primary nasal cavity, which is separated from the primary oral cavity, developed from the stomodeum, by the oronasal membrane (Fig. 14-4). With the regression of the caudal aspects of this membrane, the primary choanae connecting the developing nasal and oral cavities are formed, and the oronasal membrane itself forms the primary palate.
Construction of the facial structures around the entrance to the developing nasal and oral cavities starts from the prominences described above (Fig. 14-5). The maxillary prominence increases in size, extends further medially, and fuses with the medial nasal prominence. This lays the foundation for formation of the bones of the upper jaw (the maxilla and incisive bones) and the upper lip. The final shape of the upper lip depends upon the degree of midline fusion between the medial nasal prominences: in carnivores and small ruminants, incomplete fusion leaves a medial groove, the philtrum; in horses, cattle, and pigs, the fusion is complete and results in a continuous upper lip.
The shaping of the face varies from species to species, and even within a species, as is especially obvious in the dog (see Chapter 16). Horses, cattle, and pigs have relatively long skulls due to the growth of the bones defining the oral and nasal cavities; they are described as being dolichocephalic. Primates and humans, on the other hand, have short skulls and are brachycephalic. In the dog, some breeds are dolichocephalic, others brachycephalic, and yet others, between these extremes, are referred to as being mesocephalic.
The nasal cavity
The oronasal membrane (or primary palate) becomes replaced by the secondary palate which develops from primordia in the palate processes extending from the maxillary bones (Fig. 14-6). In parallel, the nasal septum develops from the dorsal aspect of the nasal cavity and grows ventrally. Eventually, the developing nasal septum fuses with the palate processes, partially separating the developing nasal and oral cavities (Fig. 14-7). The palate processes are membranous at first, but later intramembranous ossification in their rostral two-thirds forms the bones of the hard palate (see Chapter 16). The secondary palate closes around Day 32 of development in the cat, around Day 33 in dog and pig, between Days 49 and 56 in horse, and between Days 56 and 63 in cattle. Caudally, mesenchyme covered by the ectoderm develops into the soft palate (which is particularly long in the horse) and, in the process, the secondary choanae and the arcus palatopharyngeus are formed. Rostrally, the secondary palate fuses with the remnants of the primary palate except for the paired incisive ducts. These ducts maintain the communication between the oral and nasal cavities, except in the horse where the oral opening of the duct is obliterated by the mucosa.
Conchae
The conchae are formed from processes extending from the lateral aspect of the developing nasal cavity (see Chapter 16). They first consist of a mesenchymal core covered with ectodermal epithelium; later, endochondral ossification transforms the conchae into scroll-like structures. Conchae developed from the ethmoid bone in the caudal region of the nasal cavity form a labyrinth. The most dorsal of these, the dorsal nasal concha, is prolonged by a conchal process developing from the nasal bone and extends rostrally through the nasal cavity. Another large concha, the ventral nasal concha, develops from a conchal process on the maxilla and has no connection to the ethmoid bone.
Vomeronasal organ
As mentioned, the fusion of the secondary palate is incomplete and leaves the incisive duct as an opening between the oral and nasal cavity. A secondary duct develops in the ventral mucosa of the nasal cavity and forms the paired vomeronasal organ (Fig. 14-6). The duct is lined by ectodermal epithelium differentiating into respiratory as well as olfactory regions.
Paranasal sinuses
The ectodermal epithelium lining the nasal cavity forms solid outgrowths that penetrate the bones of the skull. The outgrowths subsequently develop a lumen and gradually form the paranasal sinuses which remain connected to the nasal cavity (see Chapter 16). The paranasal sinuses are so poorly developed at birth that they are scarcely recognizable. The final anatomy of the sinuses is species-specific and of clinical significance. For example, in the horse, the maxillary sinus is closely associated with the roots of the premolars and molars; in cattle, the frontal sinus extends into the horn processes.
The oral cavity
With the formation of the secondary palate and choanae, the nasal and oral cavities as well as the pharynx are established. Ectodermal thickenings on the developing jaws form upper and lower labio-gingival laminae. Subsequently, a loss of cells and tissues in the intermediate portions of these laminae results in the formation of lips and gums as well as the vestibulum oris between them. Lateral fusion between the upper and lower labio-gingival laminae results in development of the cheeks, thereby defining the aperture of the mouth. Caudally, the oral cavity is demarcated on each side by the palatoglossal arches extending from the border between the hard and soft palate to the root of the tongue.
Tongue
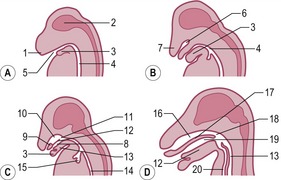
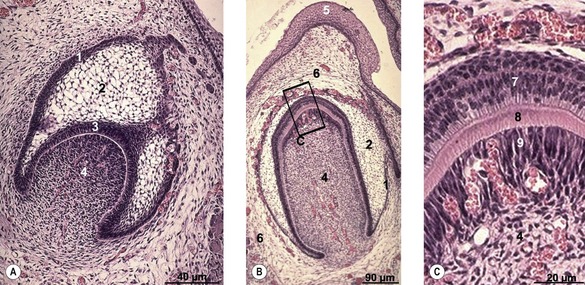
The tongue develops at the bottom of the oral cavity from a portion of the first pharyngeal arch (see later) and from myoblasts that invade the tongue primordium from the occipital myotomes. The first sign of tongue development is a medial prominence, referred to as the tuberculum impar, derived from the first pharyngeal arch (Fig. 14-8). The ingrowth that will give rise to the thyroid gland is found just caudal to this prominence (see later). Rostral and lateral to tuberculum impar, paired lateral lingual swellings develop from the first pharyngeal arch. The lateral lingual swellings fuse with each other and with the tuberculum impar. The midline fusion of the lateral swellings gives rise to the lingual septum and the lyssa in carnivores or cartilago dorsi linguae in the horse. The borders between the tuberculum impar and the lateral lingual swellings are gradually lost and the combined structures give rise to the rostral two-thirds of the tongue including its apex and body. The radix (root) of the tongue is developed from a median prominence, the copula, arising from the second pharyngeal arch and from the eminentia hypobranchialis from the third and fourth pharyngeal arch. The cupola atrophies and is overgrown by material from the rostral portion of the eminentia hypobranchialis that forms most of the root of the tongue. The muscle of the tongue is derived from occipital myotomes and is innervated by the hypoglossal nerve (XII).
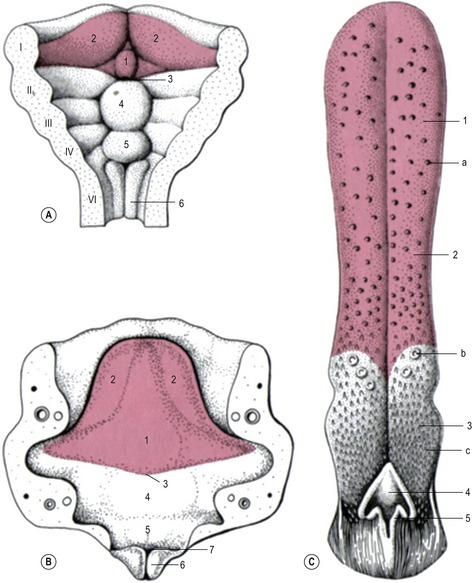
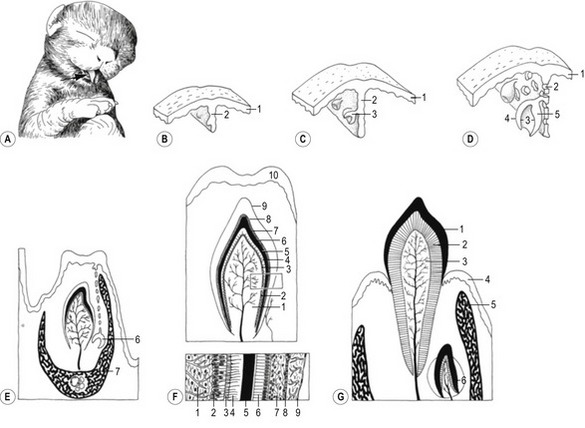
Fig. 14-8: Development of the tongue and the larynx. Red: Components developed from the first pharyngeal arch. A: I–VI: Pharyngeal arches I–VI, 1: Tuberculum impar; 2: lateral lingual swellings; 3: Primordium of the thyroid gland; 4: Copula; 5: Eminentia hypobranchialis; 6: Arytenoid swelling. B: Later developmental stages of the structures defined in A. 7: Laryngeal orifice. C: Tongue of the dog. 1: Apex linguae; 2: Corpus linguae; 3: Radix linguae; 4: Epiglottis; 5: Arytenoid; a: Papillae fungiformes; b: Papillae valatae; c: Papillae filiformes.
Courtesy Sinowatz and Rüsse (2007).
Teeth
The first step in tooth development is the formation of an ectodermal laminar ingrowth, the dental lamina, from the gingival epithelium (Fig. 14-9). Small cap-shaped dental buds develop on the side of this lamina and, with development, enclose a core of mesenchyme known as the dental papilla. Each cap-shaped dental bud has an outer and inner enamel epithelium and forms a structure known as the enamel organ (Fig. 14-10). The mesenchyme between the outer and inner enamel epithelium is known as the stellate reticulum. The shaping of the inner enamel epithelium determines the shape of the crown of the tooth.
Although formation of the enamel organ is the first step in tooth development, the first dental component to be produced is dentin. The mesenchyme adjacent to the inner enamel epithelium organizes into a columnar epithelium made up of odontoblasts. These cells produce predentin, which is deposited between the odontoblasts and the inner enamel epithelium. The predentin is subsequently mineralized and turned into bone-like dentin. However, whereas in the formation of bone osteocytes become encapsulated by the extracellular bone matrix, in tooth formation the odontoblasts withdraw from the inner enamel epithelium as increasing amounts of predentin and dentin are deposited; only long slender cytoplasmic projections from the odontoblasts remain in contact with the inner enamel epithelium and become embedded in the dentin, which is laid down concentrically around the cytoplasmic projections. Dentin production starts at the tip of the dental pulp. Odontoblasts remain active throughout life and their continuous production of predentin and dentin reduces the size of the dental pulp, which carries blood vessels and nerves.
The mesenchyme around the cap-shaped enamel organ condenses to form the dental sac. Around the root of the tooth, the mesenchyme of the dental sac differentiates into cementoblasts producing cementum, another bone-like substance similar to dentin. The cementoblasts, unlike odontoblasts, become embedded in the intercellular substance, as in bone. The more peripheral portions of the dental sac around the developing root form the tough collagen-rich fibres which become organized into the periodontal ligament that fixes the tooth in the bone of the jaw.
A first set of enamel organs is responsible for the formation of the deciduous teeth while a secondary set forms the permanent teeth (Fig. 14-9).
THE FOREGUT
The pharynx
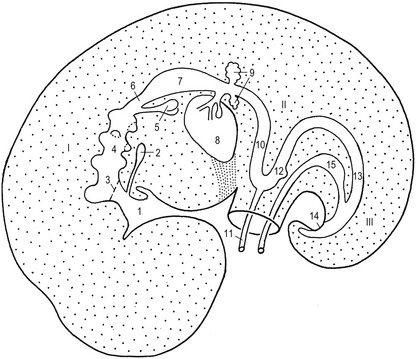
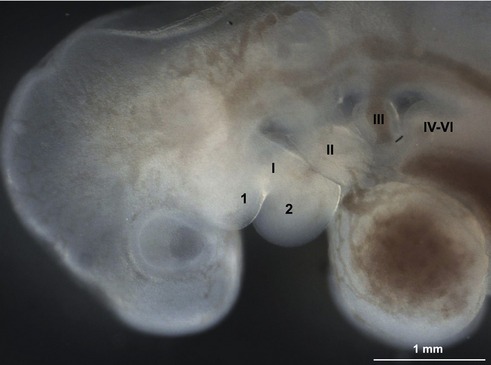
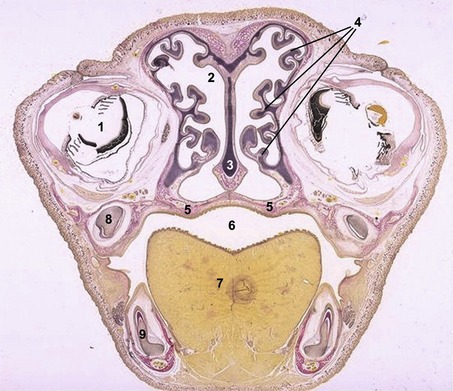
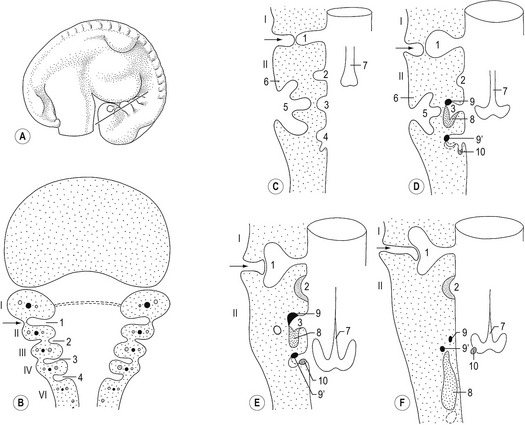
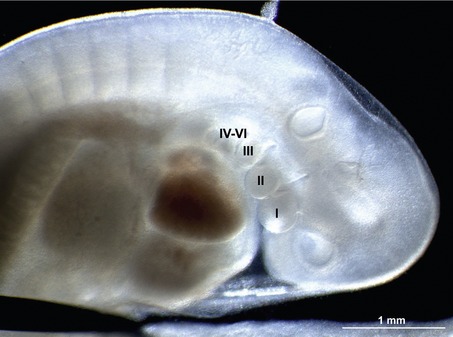
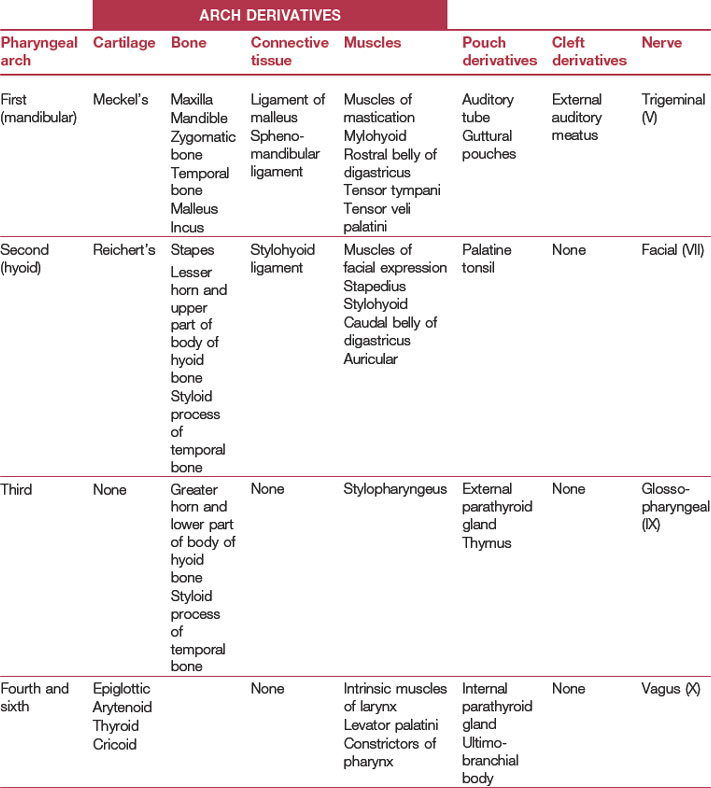
In the pharyngeal region, the foregut becomes involved in the formation of pharyngeal arches also referred to as the branchial arches from the Greek term ‘branchia’ meaning gill. In principal, six pharyngeal arches are prepared for. However, the fifth arch is rudimentary and never develops and the sixth remains part of the neck and does not become distinct. Consequently, only four pharyngeal arches are clearly visible. Just caudal to each arch, an internal pouch develops (Figs. 14-11, 14-12) and an external pharyngeal cleft corresponds to each pouch. Due to the loss of the fifth arch, the fifth pouch becomes incorporated into the fourth. Thus, often only four clefts and pouches are referred to. The pharyngeal arches and clefts/pouches are lined by ectodermal epithelium on the outside and endodermal epithelium on the inside. The mesenchyme, which is derived from neural crest cells, is thick in the arches and thin where the clefts/pouches form. However, the clefts and pouches of mammals, unlike those of fish, do not fuse to form gill slits, at least under normal conditions. The pharyngeal arches and clefts/pouches give rise to a number of organs (Table 14-1).
The pharyngeal clefts
The first pharyngeal cleft develops into the external auditory meatus (Fig. 14-11). The second, third and fourth clefts are overgrown by a caudal extension from the second pharyngeal arch. The cervical sinus is formed as a result of the initial overgrowth but closes later.
The pharyngeal arches
The pharyngeal arches consist of mesenchyme originating from the neural crest. In each arch, the mesenchyme gives rise to particular cartilage or bone derivatives, muscles, and an aortic arch (artery), and becomes associated with a particular nerve (Fig. 14-11). The arteries are described in Chapter 12.
The first pharyngeal (mandibular) arch. This arch consists of a dorsal portion, the maxillary process, and a ventral portion known as the mandibular process (Fig. 14-3). From the maxillary process come the maxilla, the zygomatic bone, and a portion of the temporal bone as well as the secondary palate by intramembranous ossification. The mandibular process contains a plate of cartilage referred to as Meckel’s cartilage. Most of this structure disappears, but its most dorsal portions give rise to the incus and malleus of the middle ear. The mandibular process also gives rise to the mandible through intramembranous ossification. The muscular derivatives of the first pharyngeal arch include the muscles of mastication (temporalis, masseter and pterygoids) as well as the mylohyoid, rostral belly of digastricus, tensor tympani, and tensor veli palatini. The first pharyngeal arch and its derivatives are supplied by the trigeminal nerve (V).
The third pharyngeal arch. The cartilage of this arch gives rise to the greater horn and lower part of the body of the hyoid bone; muscular derivatives include the stylopharyngeus; the arch and its derivatives are supplied by the glossopharyngeal nerve (IX).
The fourth and sixth pharyngeal arches. Major portions of the larynx including the epiglottic, thyroid, cricoid, and arytenoid cartilages (the latter including the corniculate and cuneiform processes) are formed from the cartilage of these arches. Derived muscles include the cricothyroid, levator palatini, constrictors of pharynx, and the remaining intrinsic muscles of larynx. The arches are supplied by the vagus nerve (X) from which the recurrent laryngeal nerve supplies all the intrinsic muscles of larynx except for the cricothyroid muscle which is supplied by the cranial laryngeal nerve. The cranial laryngeal nerve takes a direct course to the larynx at the site where the vagus nerve passes this structure. The recurrent laryngeal nerve, on the other hand, splits off from the vagus nerve much later and hooks around the sixth aortic arch on each side before making its way back cranially to the larynx (see Chapter 12, Fig. 12-15). On the right side the sixth aortic arch is obliterated, and so the recurrent nerve is released and hooks around the fourth aortic arch instead (the fifth is rudimentary). On the left, however, the sixth aortic arch is retained as the ductus arteriosus (developing postnatally into the ligamentum arteriosum) and the recurrent laryngeal nerve remains hooked around the sixth aortic arch. In the horse, this long course of the nerve on the left side may result in hemiplegia of the intrinsic laryngeal muscles in this side causing a condition referred to as ‘roaring’ where the left vocal fold is left paralyzed in the respiratory tract. The left recurrent laryngeal nerve of the giraffe is said to be the longest cell in any (land) mammal!
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree