Chapter 3 Ocular Pharmacology and Therapeutics
THERAPEUTIC FORMULATIONS
ROUTES OF ADMINISTRATION
The main factors governing choice of the route of administration are as follows:
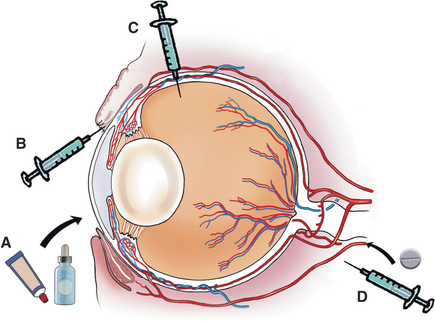
Some drugs, because of their properties, are restricted as to the routes by which they can be given. For example, polymyxin B cannot be given systemically because of nephrotoxicity or by subconjunctival injection because of local irritation. Drugs required in high concentration in the cornea or conjunctiva are usually administered by frequent topical application or subconjunctival injection. If high concentrations are required in the anterior uveal tract (i.e., iris or ciliary body), subconjunctival injection, systemic administration, or frequent topical application of drugs that will pass through the intact cornea are used. Drugs that do not pass through the blood-ocular barrier still reach high concentrations in the highly vascular anterior uvea (iris and ciliary body), posterior uvea (choroid), and sclera. With inflammation the blood-ocular barrier may be reduced, and drugs that cannot normally enter the aqueous or vitreous humor may do so. If high concentrations are required within orbital tissues, systemic administration is usually used. Choice of route is summarized in Figure 3-1.
Higher or more prolonged drug concentrations and therapeutic effects may be achieved with the following approaches:
Solutions and Suspensions (“Drops”)
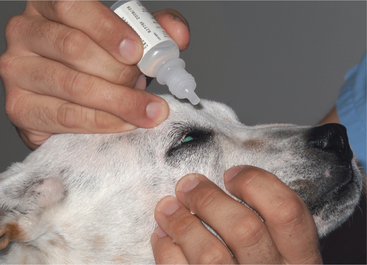
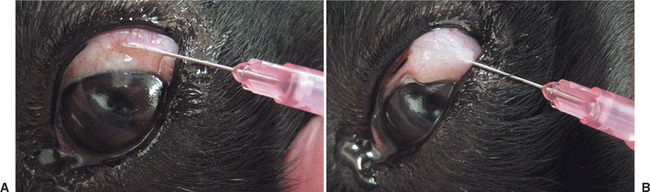
Ophthalmic solutions and suspensions (or “drops”) are commonly used for topical treatment of ocular disease. They are usually easily instilled in dogs and cats but not in large animals. The correct method for instilling eyedrops is shown in Figure 3-3. Drops permit the delivered dose to be controlled and varied easily, and they are alleged to interfere less with repair of corneal epithelium than ointments, although this last feature is unlikely to be clinically significant. Drops are quickly diluted and eliminated from the eye by tears, so greater frequency of application or drug concentration may be required, especially with increased lacrimation. It is important to note that systemic absorption of drugs from the conjunctival sac after topical application is rapid and may result in notable blood concentrations. This may be of clinical significance with use of phenylephrine (producing systemic hypertension) and long-term corticosteroid use (inducing iatrogenic hyperadrenocorticism).
Continuous or Intermittent Ocular Surface Lavage Systems
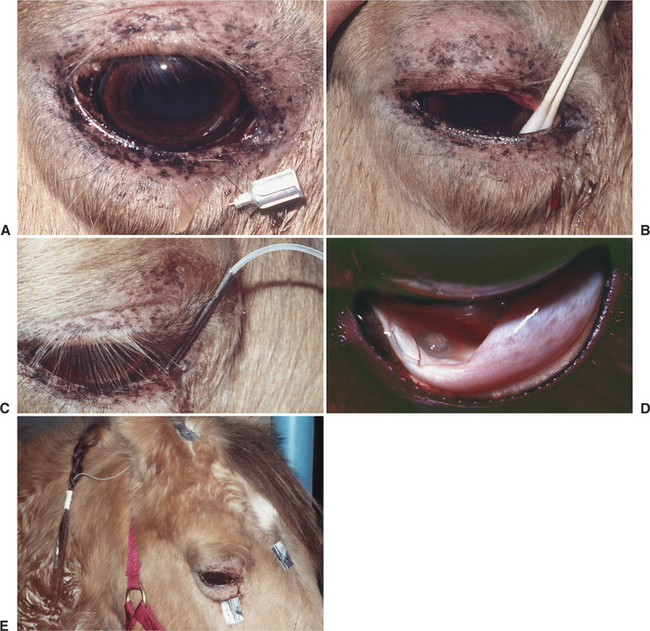
With frequent treatment or in horses with painful eyes, a lavage system allows medications to be conveniently, safely, and frequently delivered into the conjunctival sac. Originally, such systems were placed within the nasolacrimal duct and medications were instilled in a retrograde fashion. More recently subpalpebral lavage systems have been described that are simply placed and avoid nasal irritation and risk of dislodgement. A two-hole technique through the skin of the upper eyelid has now been replaced by single-hole systems, owing to the commercial availability of lavage systems with footplates to prevent inadvertent removal. The original one-hole system was placed in the central upper lid but is associated with a relatively high risk of complications, most notably corneal ulceration due to rubbing of the footplate on the cornea. Placement of the subpalpebral lavage system in the ventromedial conjunctival fornix may be preferred because of the natural corneal protection provided by the third eyelid at that point (Figure 3-4).
Ointments
In horses the orbicularis oculi muscle is very powerful, and it is impossible to tilt the head to allow a drop of solution to enter without contaminating the bottle. Ointments are preferred in such patients. Ointments also allow longer contact between the drug and surface tissues. Finally, less drug enters the nasolacrimal apparatus. Therefore ointments achieve higher tissue concentrations than solutions or suspensions do. A soothing effect occurs on instillation, they are a more stable medium for labile antibiotics and drugs, and they also provide physical lubrication and protect against desiccation better than solutions do. However, because oily ointment bases cause severe intraocular inflammation, and because application of ointments may result in ocular trauma from the tube itself, ointments are not recommended when globe perforation has occurred or is likely. Because they make tissue handling difficult and cause granulomatous inflammation if they penetrate ocular surface structures, ointments should also not be used before ocular or adnexal surgery. Finally, owners tend to overmedicate when using ointments, resulting in loss of medication, higher cost, and lower compliance with treatment regimens. As little as 0.5cm of ointment from a fine nozzle is sufficient to medicate an eye.
Subconjunctival, Subtenons, and Retrobulbar Injection
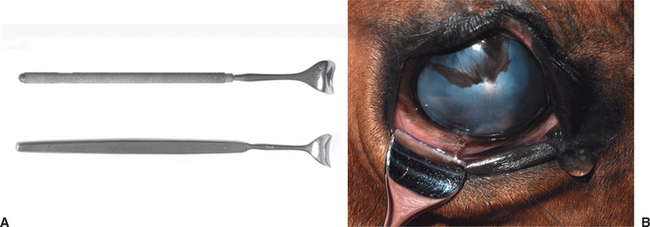
In cooperative patients, subconjunctival injections can be given using topical anesthesia only. Handheld lid retractors may be helpful in all species (Figure 3-5). For horses and cattle, one should also consider tranquilization, appropriate restraint (a twitch for horses and nose grips for cattle), and an auriculopalpebral nerve block to produce akinesia of the upper lid (see Chapter 5). A few drops of topical ophthalmic anesthetic (e.g., proparacaine) are instilled into the conjunctival sac. Conjunctival anesthesia is facilitated by a cotton-tipped applicator soaked in topical anesthesia and placed against the conjunctiva at the planned injection site for about 30 seconds. The solution for subconjunctival injection then is administered through a 25- to 27-gauge needle with a 1-mL tuberculin or insulin syringe under the bulbar conjunctiva as close as possible to the lesion being treated (Figure 3-6). Injection under the palpebral conjunctiva is not effective. The needle is rotated on withdrawal to limit leakage through the needle tract. Up to 1mL of drug can be given beneath the bulbar conjunctiva, but most injections do not exceed 0.5mL. Slight hemorrhage into the injection site occasionally occurs but is absorbed within 7 to 10 days. Injections of depot preparations should be avoided at this site because they often lead to granuloma formation.
Systemic Drug Administration
Although there are rare exceptions, systemically administered drugs should be considered to reach only the vascular tissues of the eye and surrounding structures—that is, not the cornea, the lens, or (in the presence of an intact blood-ocular barrier) the aqueous or vitreous humor. This knowledge may be used to the clinician’s advantage. For example, systemic administration of a corticosteroid for control of uveitis in the presence of corneal ulceration is safe and effective, because the target tissue (the uvea) is vascular, but the drug will not reach the avascular cornea in quantities sufficient to retard healing. Equally, the systemic administration of an antibiotic for treatment of an ulcer in a nonvascularized cornea is of little value, and topical administration is most effective. Therefore systemically administered drugs should be reserved for treatment of diseases of the eyelids, conjunctiva, sclera, uvea (iris, ciliary body, choroid), retina, optic nerve, extraocular muscles, and orbital contents.
ANTIBACTERIAL DRUGS
Antibacterial drugs act by altering cell wall synthesis, protein synthesis, or cell wall permeability in bacteria but may also have undesirable effects on the cells of the patient. Although not strictly accurate, the terms antibiotics and antibacterials often are used interchangeably. Antibacterial agents may be classified as bactericidal (destroying bacteria) or bacteriostatic (inhibiting bacterial growth and reproduction; Box 3-1). Some antibiotics may act in either manner, depending on concentration. Combining bactericidal and bacteriostatic antibiotics may result in antagonism between the agents, although the clinical importance of this effect is debated. In particular, combinations of bactericidal drugs infrequently used elsewhere in the body are commonly employed in topical treatment of the eye. This practice allows a wider spectrum of activity than with single drugs and reduces the chance of drug resistance. The combination of neomycin, polymyxin B, and bacitracin (or gramicidin) as so-called triple antibiotic is very useful. For resistant infections combinations of agents with differing mechanisms are sometimes used—for example, a penicillin or cephalosporin (which inhibits cell wall synthesis) with an aminoglycoside (which inhibits intracellular protein synthesis).
Selection and Administration of Antibiotics
The following factors must be considered in the selection of an antibiotic:
Organisms commonly isolated from the conjunctival sacs of normal and diseased animals are given in Tables 3-1 through 3-9. These tables highlight a number of important general points about ocular surface flora that are relevant when interpreting these data in an individual patient:
Table 3-1 Normal Flora of the Canine Conjunctival Sac
| AREA AND FLORA | PERCENTAGE OF CASES WITH POSITIVE CULTURES |
|---|---|
| WESTERN UNITED STATES* | |
| Diphtheroids | 75.0 |
| Staphylococcus epidermidis | 46.0 |
| Staphylococcus aureus | 24.0 |
| Bacillus spp. | 12.0 |
| Gram-negative organisms (Acinetobacter, Neisseria, Moraxella, and Pseudomonas spp.) | 7.0 |
| Streptococcus spp. (α-hemolytic) | 4.0 |
| Streptococcus spp. (β-hemolytic) | 2.0 |
| MIDWESTERN UNITED STATES† | |
| S. epidermidis | 55.0 |
| S. aureus | 45.0 |
| Streptococcus spp. (α-hemolytic) | 34.0 |
| Diphtheroids | 30.0 |
| Neisseria spp. | 26.0 |
| Pseudomonas spp. | 14.0 |
| Streptococcus spp. (β-hemolytic) | 7.3 |
| EASTERN AUSTRALIA‡ | |
| S. aureus | 39.0 |
| Bacillus spp. | 29.0 |
| Corynebacterium spp. | 19.0 |
| S. epidermidis | 16.0 |
| Yeasts | 5.0 |
| Streptococcus spp. (α-hemolytic) | 3.0 |
| Streptococcus spp. (nonhemolytic) | 3.0 |
| Micrococcus spp. | 3.0 |
| Neisseria spp. | 2.0 |
| Streptococcus spp. (β-hemolytic) | 1.0 |
| Pseudomonas spp. | 1.0 |
| Nocardia spp. | 1.0 |
| Escherichia coli | 1.0 |
| Clostridium spp. | 1.0 |
| Enterobacter spp. | 1.0 |
| Flavobacterium spp. | 1.0 |
| Branhamella catarrhalis | 1.0 |
* Data from Bistner SI, et al. (1969): Conjunctival bacteria: clinical appearances can be deceiving. Mod Vet Pract 50:45.
† Data from Urban W, et al. (1972): Conjunctival flora of clinically normal dogs. Am J Vet Med Assoc 161:201.
‡ Data from McDonald PJ, Watson ADJ (1976): Microbial flora of normal canine conjunctivae. J Small Anim Pract 17:809.
Table 3-2 Organisms Cultured from Dogs with External Ocular Disease
| AREA AND FLORA | PERCENTAGE OF CASES WITH POSITIVE CULTURES |
|---|---|
| THE NETHERLANDS* | |
| Streptococcus canis | 20.3 |
| No growth | 18.7 |
| Staphylococcus epidermidis | 14.0 |
| Staphylococcus aureus | 7.8 |
| Other nonpathogenic Streptococcus spp. | 7.8 |
| Nocardia spp. | 7.8 |
| Absidia ramosa (fungus) | 7.8 |
| Pseudomonas aeruginosa | 6.1 |
| Corynebacterium spp. | 4.6 |
| Other pathogenic S. canis spp. | 3.1 |
| Proteus vulgaris | 3.1 |
| Clostridium perfringens | 1.5 |
| Candida spp. | 1.5 |
| COLORADO† | |
| S. aureus | 68.0 |
| S. epidermidis | 27.0 |
| Streptococcus spp. (β-hemolytic) | 19.0 |
| Streptococcus spp. (α-hemolytic) | 17.0 |
| Proteus mirabilis | 11.0 |
| Escherichia coli | 10.0 |
| Bacillus spp. | 5.0 |
| Corynebacterium spp. | 3.0 |
| P. aeruginosa | 2.0 |
| Klebsiella spp. | 1.0 |
| ILLINOIS‡ | |
| Staphylococcus spp. | 39.4 |
| Coagulase positive | 29.0 |
| Staphylococcus intermedius | 17.0 |
| S. epidermis | 11.0 |
| Streptococcus spp. | 25.2 |
| β-Hemolytic streptococci | 17.0 |
| Pseudomonas spp. | 9.4 |
* Data from Verwer MAJ, Gunnick JW (1968): The occurrence of bacteria in chronic purulent eye discharge. J Small Anim Pract 9:33.
† Data from Murphy JM, et al. (1978): Survey of conjunctival flora in dogs with clinical signs of external eye disease. J Am Vet Med Assoc 172:66.
‡ Data from Gerding PA, et al. (1988): Pathogenic bacteria and fungi associated with external ocular diseases in dogs: 131 cases (1981-1986). J Am Vet Med Assoc 193:242.
Table 3-3 Normal Flora of the Feline Conjunctival Sac
| PERCENTAGE OF CASES WITH POSITIVE CULTURES | ||
|---|---|---|
| AREA AND FLORA | CONJUNCTIVA | LIDS |
| WESTERN UNITED STATES | ||
| Staphylococcus epidermidis | 16.3 | 13.3 |
| Staphylococcus aureus | 10.4 | 8.8 |
| Mycoplasma spp. | 5.0 | — |
| Bacillus spp. | 2.9 | 1.7 |
| Streptococcus spp. (α-hemolytic) | 2.5 | 1.7 |
| Corynebacterium spp. | 1.3 | — |
| Escherichia coli | — | 0.4 |
Data from Campbell L (1973): Ocular bacteria and mycoplasma of the clinically normal cat. Feline Pract Nov-Dec:10.
Table 3-4 Fungal Flora from Normal Horses (50 Horses)
| ORGANISM | PERCENTAGE OF CASES WITH POSITIVE CULTURES |
|---|---|
| Aspergillus spp. | 36.0 |
| Cladosporium spp. | 34.0 |
| Positive but no quantitative data given: Alternaria, Fusarium, Monotospira, Paecilomyces, Phoma, Pullularia, Scopulariopsis, Streptomyces, Trichoderma, Verticullium spp. | |
Modified from Smith PJ, et al. (1997): Identification of sclerotomy sites for posterior segment surgery in the dog. Vet Comp Ophthalmol 7:180.
Table 3-5 Organisms Cultured from Horses with External Ocular Disease (123 Eyes)
| ORGANISM | PERCENTAGE OF CASES WITH POSITIVE CULTURES |
|---|---|
| Streptococcus spp. (total) | 43.9 |
| β-Hemolytic | 26.0 |
| Other hemolytic | 17.9 |
| Staphylococcus spp. | 24.4 |
| Pseudomonas spp. | 13.8 |
| Bacillus spp. | 10.6 |
| Enterobacter spp. | 6.5 |
| Escherichia coli | 4.0 |
| Corynebacterium spp. | 3.2 |
| Proteus spp. | 3.2 |
| Aspergillus spp. | 2.4 |
| Klebsiella spp. | 2.4 |
| Moraxella spp. | 2.4 |
| Pasteurella spp. | 2.4 |
| Mima spp. | 1.6 |
| Diplococcus spp. | 0.8 |
| Flavobacterium spp. | 0.8 |
| Fusarium spp. | 0.8 |
| Neisseria spp. | 0.8 |
| Nocardia spp. | 0.8 |
| Penicillium spp. | 0.8 |
| Rhizopus spp. | 0.8 |
| Trichosporon spp. | 0.8 |
Data from McLaughlin SA, et al. (1983): Pathogenic bacteria and fungi associated with extraocular disease in the horse. J Am Vet Med Assoc 182:241.
Table 3-6 Normal Flora of the Bovine Conjunctival Sac
| AREA AND FLORA | PERCENTAGE OF CASES WITH POSITIVE CULTURES |
|---|---|
| NORTHEASTERN AUSTRALIA | |
| Unidentified gram-positive cocci | 54.4 |
| Corynebacterium spp. | 27.4 |
| Moraxella nonliquefaciens | 26.9 |
| Streptococcus faecalis | 20.0 |
| No growth | 13.4 |
| Neisseria (Branhamella) catarrhalis (nonhemolytic) | 10.5 |
| Unidentified gram-negative rods | 8.5 |
| Acinetobacter spp. | 8.0 |
| Moraxella bovis | 6.5 |
| Coliforms | 6.5 |
| Staphylococcus aureus | 4.1 |
| Moraxella liquefaciens | 2.2 |
| Bacillus spp. | 1.3 |
| Unclassified Moraxella | 1.0 |
| Actinobacillus spp. | 0.7 |
| Proteus spp. | 0.0 |
Data from Wilcox G (1970): Bacterial flora of the bovine eye with special reference to Moraxella and Neisseria. Aust Vet J 46:253.
Table 3-7 Normal Flora of the Ovine Conjunctival Sac
| AREA AND FLORA | PERCENTAGE OF CASES WITH POSITIVE CULTURES |
|---|---|
| EASTERN AUSTRALIA | |
| No growth | 60.0 |
| Neisseria ovis | 24.0 |
| Micrococcus spp. | NQD |
| Streptococcus spp. | NQD |
| Corynebacterium spp. | NQD |
| Achromobacter spp. | NQD |
| Bacillus spp. | NQD |
| Moraxella spp. | NQD |
NQD, No quantitative data (present in small numbers, but NQD given).
Data from Spradbrow P (1968): The bacterial flora of the ovine conjunctival sac. Aust Vet J 44:117.
Table 3-8 Normal Bacterial Flora of South American Camelid Eyes (88 Animals)
| ORGANISM | PERCENTAGE OF CASES WITH POSITIVE CULTURES |
|---|---|
| GRAM-POSITIVE | |
| Staphylococcus spp. | 58 |
| Bacillus spp. | 28 |
| Streptomyces spp. | 18 |
| α-Hemolytnoic Streptococcus spp. | 13 |
| Corynebacterium spp. | 8 |
| Micrococcus/Planococcus spp. | 7 |
| GRAM-NEGATIVE | |
| Pseudomonas spp. | 41 |
| Pasteurella ureae | 9 |
| Klebsiella spp. | 2 |
| Escherichia coli | 2 |
Data include llama, alpaca, and guanaco eyes and are from Gionfriddo JR, et al. (1991): Bacterial and mycoplasmal flora of the healthy camelid conjunctival sac. Am J Vet Res 52:1061.
Table 3-9 Normal Fungal Flora of South American Camelid Eyes (127 Animals)
| ORGANISM | PERCENTAGE OF CASES WITH POSITIVE CULTURES* |
|---|---|
| Aspergillus spp. | 20-43 |
| Fusarium spp. | 2-30 |
| Rhinocladiella spp. | 8-35 |
| Penicillium spp. | 3-33 |
| Mucor spp. | 5-14 |
| Dematiaceous fungi | 12-33 |
* Percentages varied according to season and camelid species.
Data include llama, alpaca, and guanaco eyes and are from Gionfriddo JR, et al. (1992): Fungal flora of the healthy camelid conjunctival sac. Am J Vet Res 53:643.
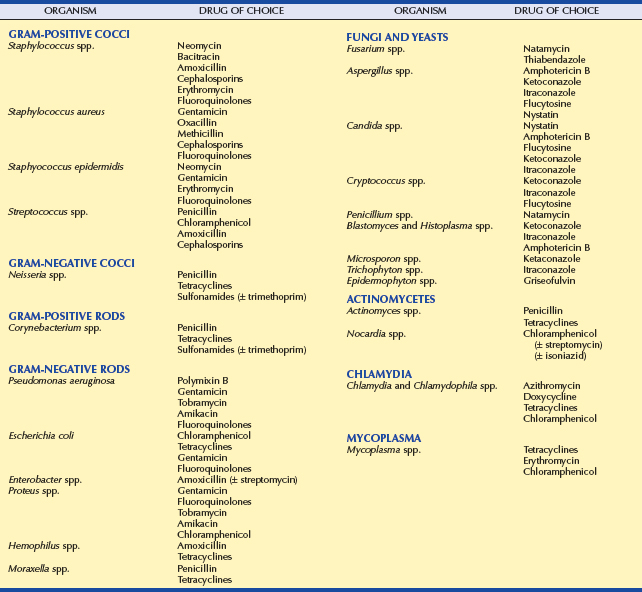
The following sections provide a summary of some important properties of antibiotics used commonly in veterinary ophthalmology, and Table 3-10 lists the typical Gram-staining characteristics of, and the antibiotics of choice for, common organisms.
Penicillins
Sodium Methicillin
Methicillin is resistant to β-lactamase and is used by intravenous infusion for resistant staphylococci. Renal excretion is rapid. Because the drug is unstable in solution, it should be dissolved just before use. Methicillin can be used topically or by subconjunctival injection for corneal infections. Methicillin may be expected to enter the aqueous humor in therapeutic concentrations when the blood-ocular barrier is disrupted by inflammation.
Chloramphenicol
Chloramphenicol is a broad-spectrum bacteriostatic antibiotic effective against a wide range of gram-positive and gram-negative organisms, Rickettsia, spirochetes, and Chlamydophila spp. However, Pseudomonas aeruginosa is often resistant. Because of its lipid solubility, chloramphenicol passes the blood-ocular barrier and through corneal epithelium better than most water-soluble antibiotics. Chloramphenicol may be administered orally, intramuscularly, subcutaneously, intravenously, subconjunctivally, or topically. Because absorption after oral administration results in high blood concentrations, this is the route of choice for infections in the posterior globe and orbit. Despite controversial toxicity studies in cats, the drug has been used clinically over many years with few ill effects except anorexia and occasional pyrexia in some cats after systemic administration, provided that administration is not prolonged. Because of its wide spectrum of activity and intraocular penetration, chloramphenicol as an ointment or drops was widely used in veterinary practice for ocular surface injuries and infections, but its use is declining in favor of bactericidal antibiotics. Polymyxin B is often added to aid in control of gram-negative organisms, especially Pseudomonas spp. It is still useful in the topical treatment of suspected Chlamydophila infections in cats, although systemically administered drugs are preferable (see Chapter 7).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree