Chapter 13 Ocular Manifestations of Systemic Disease
Neuro-ophthalmic Diseases
Equine Protozoal Myeloencephalitis
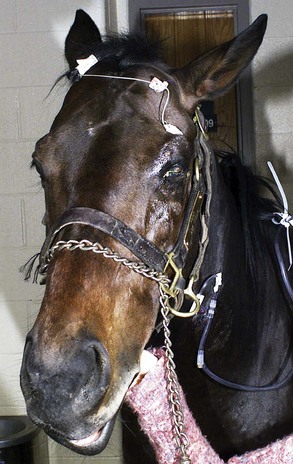
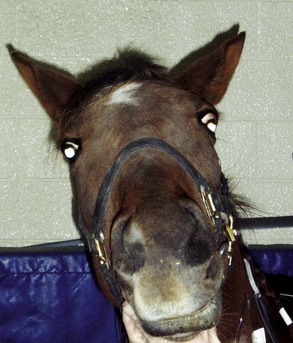
To date, there are no reported visual deficits associated with equine protozoal myeloencephalitis (EPM, Sarcocystis neurona), although cerebral lesions could result in blindness.1 No fundic lesions were seen in an extensive unpublished evaluation of EPM cases (M. Willis, personal communication, 2003). However, if EPM affects the brainstem, cranial nerves (CNs) VII and VIII are usually involved, resulting in facial nerve paralysis (Box 13-1) and vestibular disease.1 Ocular clinical signs associated with facial nerve paralysis include ptosis, absence of the menace response, and absence of the palpebral reflex (Fig. 13-1). If the parasympathetic nucleus of CN VII is affected, neurogenic keratoconjunctivitis sicca will occur, predisposing the cornea to ulceration and secondary infectious keratitis. Involvement of CN III, IV, and VI may result in unilateral strabismus (Fig. 13-2). The presence of ventrolateral strabismus plus the absence of a pupillary light reflex (PLR) in the stimulated eye, with a normal consensual PLR, indicate an ipsilateral lesion in the oculomotor nerve. If ocular manifestations are present, they are usually accompanied by other cranial nerve deficits. EPM affecting the cervical spinal cord can cause Horner’s syndrome (Box 13-2),2 and clinical signs are listed in the discussion of Horner’s syndrome. Other clinical signs associated with EPM include ataxia, (often asymmetrical and worse in the hind limbs), weakness, and muscle atrophy.1 Some horses may have acute paraplegia or tetraplegia associated with periods of stress such as shipping, pregnancy, or concurrent illness.3 Diagnosis of EPM is based on typical clinical signs, exclusion of other disease processes, plus the results of immunodiagnostic testing. The most common and reliable immunodiagnostic tests include Western blot or indirect fluorescent antibody test (IFAT) on serum or cerebrospinal fluid (CSF).4 Positive results on serum titers are only indicative of exposure and cannot be used to diagnose central nervous system (CNS) disease; however, a negative result remains a good means of ruling out EPM.5 Positive results on CSF may result from contamination of the sample with red blood cells, so results should be interpreted with caution.5 One advantage of the IFAT test is that it provides an actual quantitative titer, with serum titers greater than 1:100 and CSF titers greater than 1:5 being more indicative of active disease. Intrathecal production of antibodies without actual invasion of the CNS, or serum-derived antibodies crossing the blood-brain barrier (as occurs with vaccinated horses) are also potential causes of false-positive results. There is no one single ideal test for EPM; the best means of ruling out the disease is a negative result for serum or CSF.
Box 13-1 | Systemic Illness Causing Facial Nerve Dysfunction
Numerous treatments are licensed in the United States for the treatment of EPM.6 A combination product of sulfadiazine and pyrimethamine (ReBalance Antiprotozoal Oral Suspension [IVX Animal Health Inc., St. Joseph, MO]) has been used successfully, although treatment is often prolonged, and bone marrow suppression may occur. Newer antiprotozoal drugs include diclazuril, toltrazuril, and ponazuril. Ponazuril (Marquis Antiprotozoal Oral Paste [Bayer Corporation, Shawnee Mission, KS]) is available in the United States and comes in a paste formulation for easy administration. Treatment for 1 or 2 months with these drugs is recommended. Nitazoxanide (Navigator Antiprotozoal Oral Paste [IDEXX Pharmaceuticals, Greensboro, NC]) has also been shown to be effective for the treatment of EPM, but this drug is not often first-line treatment, owing to its potential for causing severe gastrointestinal side effects. Regardless of which treatment is used, approximately 65% of horses show an improvement in neurologic scores of one to two grades.1
Equine Leukoencephalomalacia
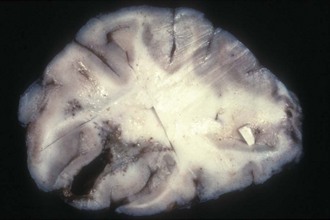
Blindness (Box 13-3) caused by equine leukoencephalomalacia, also called blind staggers or moldy corn disease, occurs when the midbrain (cortex-cerebrum) is affected, resulting in central blindness.7,8 Severe neurologic changes, ataxia, and manic behavior will often accompany the blindness associated with equine leukoencephalomalacia. The disease is caused by chronic ingestion of moldy corn infested with Fusarium moniliforme and its associated toxin, fumonisin B1.9,10 It results in liquefactive necrosis of the white matter in the brain (Fig. 13-3). If one horse on the farm is affected, the other horses have most likely been exposed, and the feed should be checked for presence of the mold. Hepatic enzyme activities—in particular, activity of gamma-glutamyl transferase—are often high in horses exposed to the toxin, and this can be used as a screening test for other horses on the farm.11 Once neurologic signs have developed, the prognosis is grave. If treatment is attempted, it should consist of supportive care, provision of fluids, and administration of activated charcoal to decrease absorption of the toxin.
Box 13-3 | Equine Leukoencephalomalacia
Equine Motor Neuron Disease
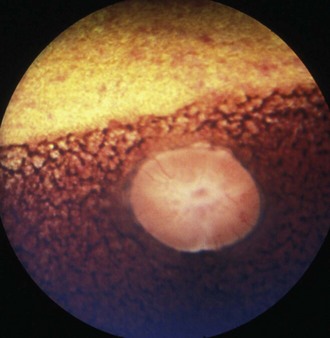
Visual deficits are not usually reported with equine motor neuron disease, but approximately 50% of horses with equine motor neuron disease12 will have characteristic fundic changes caused by ceroid/lipofuscin accumulation in the retinal pigment epithelium, considered pathognomonic for the disease. This dark brown to yellow pigment is deposited in a honeycomb mosaic pattern in the area of the tapetal fundus, with a distinct band of pigment at the tapetal/nontapetal junction (Fig. 13-4). PLRs may be abnormal.13
Severe weakness and muscle atrophy and fasciculations are associated with denervation atrophy of type I muscle fibers.14 Affected horses are not ataxic, and clinical signs actually improve when horses are in motion.14 The disease usually affects older horses and is slowly progressive. Decreased serum vitamin E levels have been associated with an increased risk of developing the disease and may be related to the pathophysiology, as evidenced by oxidative injury to the spinal cord, particularly in the somatic ventral motor neuron cells.15,16 Diagnosis is often made on the basis of clinical signs, decreased serum vitamin E levels, increased serum ferritin and hepatic iron concentrations, and findings from electromyography and biopsy of the spinal accessory nerve or sacrodorsalis caudalis muscle.15–17
Eastern Equine Encephalomyelitis, Western Equine Encephalomyelitis, and Venezuelan Equine Encephalomyelitis
Togaviral encephalitides are transmitted by mosquitoes and tend to have a typical geographic distribution according to antigenic variation.18 Typical clinical signs include fever, conscious proprioceptive deficits, and behavioral changes. Viremia occurs during this period. In the later stages of the disease, progressive signs of cerebral or cerebrocortical and cranial nerve dysfunction can be seen, including blindness; facial, lingual, and pharyngeal paralysis; ataxia; hypermetria; circling; head pressing (Fig. 13-5); and proprioceptive deficits or paresis of the trunk and limbs (see Boxes 13-1 and 13-3).19 When the brainstem becomes involved, strabismus, nystagmus, pupil dilatation, and head tilt ensue.20 Diagnosis is based on clinical signs, viral titers, and often, postmortem findings. CSF cytology may reveal a neutrophilic inflammation, and histopathologic examination reveals a nonseptic suppurative inflammatory infiltrate. An immunoglobulin (Ig) M capture enzyme-linked immunosorbent assay (ELISA) may be used to differentiate actual infection from vaccine responses.21 Treatment involves supportive therapy with fluids, antiinflammatory drugs, and management of the recumbent horse. The mortality rate for Eastern equine encephalitis is the highest and is reported to be 75% to 100%, compared with the mortality rates for Venezuelan equine encephalitis at 40% to 80% and Western equine encephalitis at 20% to 50%.18 If the horse does recover from the acute phase of the disease, residual neurologic deficits are common, especially with Eastern equine encephalitis. A vaccine that provides adequate protection for 6 to 8 months is available.22
Septic Meningoencephalitis
Septic meningoencephalitis is rare in adult horses but more common in young foals. In the neonate, hematogenous spread is the most common cause of the disease, but in the adult, it is often a direct extension of infectious agents into the CNS as a result of skull fractures, sinusitis, otitis, or surgical procedures.23,24 Hematogenous spread may also occur in adult horses as a result of bacterial endocarditis or respiratory disease.23,25 Clinical signs in foals often begin with stiffness in the neck, photophobia, and hyperesthesia. These signs can then rapidly progress to behavioral changes, cranial nerve deficits, ataxia, coma, seizures, and death. Signs are similar in adult horses but tend to progress slowly over a period of several days.26 Specific ocular signs that have been reported in horses with bacterial meningoencephalitis include blindness (see Box 13-3), lack of a menace response, lack of a pupillary light response, nystagmus, ventromedial strabismus, decreased palpebral reflex, retraction of the globe, and elevation of the third eyelid.25,26 Lesions have been reported to be unilateral or bilateral and result from inflammation of the cerebral cortex or individual cranial nerves.25,26 Diagnosis is made on the basis of findings from CSF cytology, Gram staining, and culture. Treatment can be difficult because of the well-developed blood-brain barrier. Antibiotic choices should be based on findings from culture and sensitivity testing, as well as the physicochemical properties of the drug. In general, lipophilic, nonpolar basic drugs with a broad spectrum of activity and bactericidal action should be considered. In horses that do not respond to typical antibiotic therapies, nonbacterial causes of meningitis should be considered. Fungal infections with Cryptococcus neoformans27 and parasitic infections with Halicephalobus gingivalis28 have also been reported to cause meningitis with concurrent blindness in the horse. Antiinflammatory medications and anticonvulsants are also necessary. The prognosis should be considered guarded in all cases.
Thiamine Deficiency
Thiamine deficiency in the horse can be induced by feeding a diet deficient in thiamine or by the ingestion of thiaminase-containing plants, such as bracken fern (Pteridium aquilinum) and horsetail (Equisetum arvense).7,23 Amprolium has been used experimentally to induce thiamine deficiency.29 Ataxia, conscious proprioceptive deficits, heart block, bradycardia, blindness, and muscle fasciculations or rhabdomyolysis have all been reported. Electrolyte changes, hyperglycemia, decreased glucose tolerance, and high creatine kinase activities have also been described.23,30 Supplementation with injectable thiamine is successful in the early stages of the disease.23 Clinical signs, including blindness, are reversible in the earlier stages of the deficiency.7
Vestibular Disease
Vestibular disease can be classified as peripheral or central. Peripheral vestibular disease causes head tilt toward the side of the lesion; horizontal or rotary nystagmus, with the fast phase occurring away from the side of the lesion23; falling; circling; and asymmetric ataxia without conscious proprioceptive deficits or weakness. Facial nerve paralysis can occur with peripheral disease because of the facial nerve’s proximity to the petrous temporal bone. Ipsilateral Horner’s syndrome occurs when the postganglionic sympathetic nerves are affected as they course through the petrous temporal bone. Common causes of peripheral vestibular dysfunction include trauma, otitis media, temporohyoid osteoarthropathy, and guttural pouch disease.31,32
Central vestibular disease presents in a similar manner; however, conscious proprioceptive deficits, generalized weakness, involvement of multiple cranial nerves, and nystagmus may also be present. Nystagmus may be indistinguishable from peripheral disease but may also be vertical, diagonal, or disconjugate (different in each eye). The direction of the nystagmus may change with head position in horses with central vestibular disease.33 Paradoxical central vestibular disease may occur with destructive lesions of the cerebellopontine angle or the flocculonodular lobe, resulting in clinical signs contralateral to the lesion.34 The nuclei of CNs V to XII can be involved; therefore cranial nerve dysfunction, besides dysfunction of CN VII, indicates central disease. In addition, Horner’s syndrome is not seen with central vestibular disease. Bilateral vestibular disease is difficult to differentiate from generalized cerebellar disease, and horses with bilateral vestibular disease do not exhibit typical clinical signs. Central vestibular dysfunction is commonly associated with space-occupying lesions such as tumors or abscesses but may also be caused by protozoal, viral, bacterial, or parasitic encephalitides.31
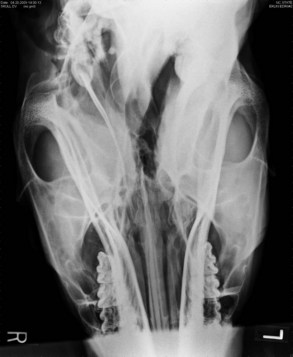
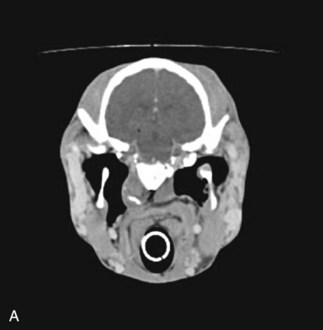
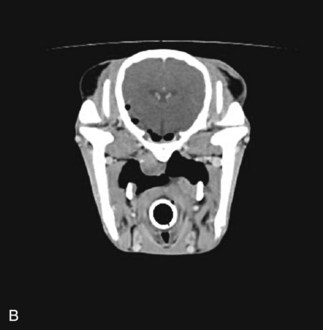
Diagnostic techniques for determining the underlying cause of vestibular disease in the horse should include radiographs of the skull (Fig. 13-6), endoscopy of the pharyngeal region and guttural pouches, and possibly magnetic resonance imaging (MRI) or a computed tomography (CT) scan of the head (Fig. 13-7). CSF cytology and ancillary testing for viral or protozoal antibodies are indicated if central disease is suspected. Caloric testing by irrigating the ear canal with cold water and checking for induction of normal horizontal nystagmus can be done, although the test is not always reliable, and most horses will resist the procedure.35 A decreased or absent reaction indicates the side of the lesion. Brainstem auditory evoked responses are also useful in demonstrating damage to the cochlea and CN VIII and can be used to differentiate between central and peripheral disease.36,37 The procedure is reliable and repeatable in the sedated horse. Treatment and prognosis should be related to the primary disease process.
Horner’s Syndrome
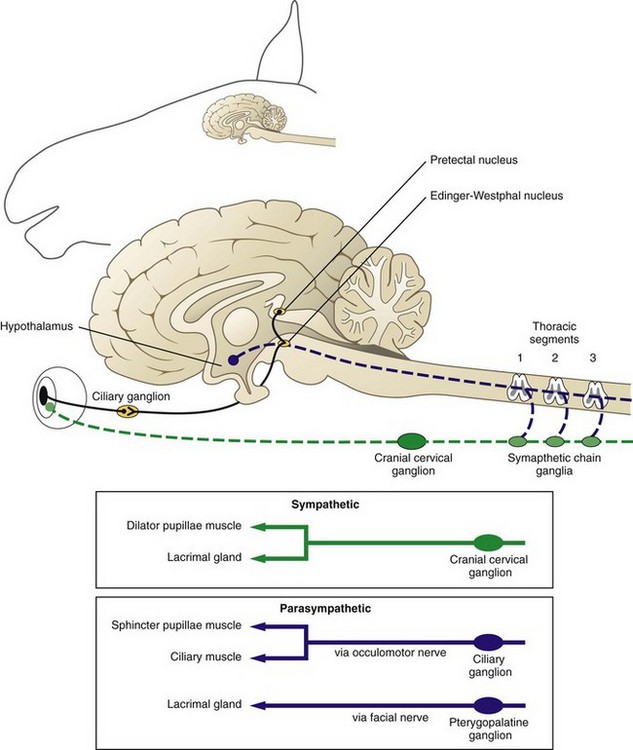
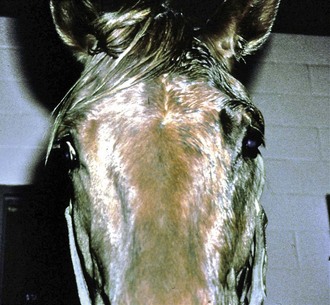
Horner’s syndrome is caused by damage or denervation along any portion of the sympathetic nervous system. The sympathetic nervous system originates in the hypothalamus (Fig. 13-8). The central sympathetic fibers form the tectotegmentospinal tract (first-order neurons), which descends ipsilaterally through the brainstem and lateral funiculus of the cervical spinal cord to synapse with the preganglionic cell bodies of T1 to T3 or T4. These preganglionic sympathetic neurons (second-order neurons) leave the spinal cord via the segmental ventral roots to the paravertebral sympathetic chain, continue through the brachial plexus, then travel with the vagosympathetic trunk until they synapse at the cranial cervical ganglion caudomedial to the tympanic bulla. The postganglionic fibers (third-order neurons) join the tympanic branch of CN IX (glossopharyngeal nerve) within the middle ear and, in horses, pass over the caudodorsal aspect of the guttural pouch. After the fibers exit the middle ear, they enter the cavernous sinus and join CN V and together continue rostrally in the periorbita (nasociliary nerve) to innervate the orbital smooth muscles, the eyelids (including the third eyelid), and the ciliary body, iris dilator, and iris sphincter muscles.38,39 Horner’s syndrome in horses is most frequently seen as a result of guttural pouch diseases that involve the internal carotid nerve, which is composed of postganglionic sympathetic fibers. Injury to the cranial thoracic spinal cord, brachial plexus avulsions, traumatic lesions, or masses (tumors or abscesses) involving the mediastinum, periorbital tissues, or cervical structures may also cause clinical signs of Horner’s syndrome. Iatrogenic Horner’s syndrome can occur as a result of ligation of the carotid artery.39 Causes unique to the horse, besides guttural pouch infections, include polyneuritis equi syndrome (cauda equina neuritis), EPM affecting the cervical spinal cord, basisphenoid trauma, esophageal rupture, and extravasation of an intravenous drug (e.g., phenylbutazone).40–42 Clinical signs of Horner’s syndrome in horses include ptosis, relative enophthalmos, subtle miosis (anisocoria), regional hyperthermia, and excessive sweating on the ipsilateral side of the face (Fig. 13-9).40 Cervical sympathetic nerve damage will cause sweating of the neck, congested conjunctival and nasal mucous membranes, and inspiratory stridor. Regional hyperthermia and sweating are thought to be caused by decreased vasomotor tone resulting in vasodilation and increased cutaneous blood flow.43–45
The location and cause of the lesion will determine which of the clinical signs are present, and the location will determine what diagnostic tests should be performed. Often, location may provide the prognosis. Phenylephrine (2% or 10%) or 1:1000 epinephrine is more readily available than hydroxyamphetamine or cocaine for the pharmacologic localization of efferent sympathetic lesions. In addition, although cocaine will confirm the presence of a sympathetic deficit, it will not localize the lesion. Hydroxyamphetamine is an indirect-acting sympathomimetic agent that causes endogenous release of norepinephrine from the adrenergic nerve endings. After instillation of topical hydroxyamphetamine, central and preganglionic lesions will have normal mydriasis, and postganglionic lesions will have incomplete to no mydriasis compared with the normal eye. Phenylephrine and epinephrine are direct-acting sympathomimetic drugs that will result in mydriasis within 5 to 8 minutes in postganglionic lesions. This is due to denervation hypersensitivity of the effector cells.39 One drop of phenylephrine or epinephrine is applied to each eye (the normal eye is used for comparison), and care should be taken to use the same amount in each eye because the response is dose dependent. If hydroxyamphetamine is used before administration of phenylephrine or epinephrine, a 24-hour washout period between the two tests is necessary. If a lesion localizes to the postganglionic arm of the pathway, endoscopy of the pharynx and guttural pouch should be performed.39 CT is another diagnostic option when it is available.
Tetanus
Tetanus most often occurs after infection of a wound with Clostridium tetani, an anaerobic, motile, gram-positive, nonencapsulated spore-forming bacteria ubiquitous in soils worldwide.39,46 The bacteria produce at least three toxins responsible for the clinical signs of the disease: tetanospasmin, tetanolysin, and a nonspasmogenic toxin. Tetanospasmin is responsible for the clinical signs typically associated with tetanus and is thought to work by inhibiting release of glycine and gamma-aminobutyric acid, the main inhibitory neurotransmitters in the CNS.23 Tetanolysin causes local tissue necrosis, which helps promote bacterial proliferation, and the nonspasmogenic toxin produces overstimulation of the sympathetic nervous system.
The characteristic ocular sign in horses is “flashing” third eyelids (Box 13-4). Flashing refers to the rapid protrusion and retraction of the third eyelids coupled with eyeball retraction, usually in response to a menacing gesture.39 Concurrent clinical signs often include lameness and a stiff gait that progresses to generalized spasticity and severe muscular rigidity. Sensory stimulation can produce muscle spasms, leading to recumbency and the sawhorse stance typical of tetanus. Ocular signs of advanced tetanus include ventrolateral strabismus and fixed, dilated pupils with normal vision.39 Death usually occurs as a result of involvement of the respiratory tract muscles, leading to hypoxemia, and cardiac failure caused by systemic hypertension.47 The principles of therapy center around providing muscle relaxation through the use of phenothiazine tranquilizers, barbiturates, or benzodiazepines; preventing self-trauma by providing adequate bedding and good footing; decreasing external stimulation; and preventing further toxin absorption or binding by cleaning and débriding the wound, administration of appropriate antibiotics including high doses of penicillin, and administration of commercially available tetanus antitoxin locally in the wound, intramuscularly, or intrathecally. The prognosis for horses with tetanus is poor, with a mortality rate of 80%. Prevention with annual administration of a tetanus toxoid vaccine is safe and effective and should be recommended for all horses on a routine basis and especially before surgical procedures. Administration of tetanus antitoxin has been associated with the development of acute necrotic hepatitis (Theiler’s disease), and its use should be limited to horses at high risk for developing tetanus and those showing clinical signs.48
Botulism
The clinical signs of botulism are caused by a neurotoxin elicited from the anaerobic bacteria, Clostridium botulinum. The toxin blocks the release of acetylcholine from the presynaptic peripheral cholinergic neurons, resulting in a neuromuscular blockade and generalized muscular weakness.49 Botulinum toxin is considered to be one of the most potent known toxins, and horses are more sensitive to the toxin than most other species. Three causes of botulism occur in horses: ingestion of preformed toxin, ingestion of spores (toxicoinfectious botulism or shaker foal syndrome), and contamination of a wound.
Ocular signs associated with botulism include enophthalmos caused by retractor bulbi spasm, upper eyelid ptosis,50 and mydriasis with slow PLRs.51 Aside from the ocular signs, horses may be presented with limb weakness and ataxia that begins caudally and progresses cranially, decreased tail and anal tone, bladder paralysis, weakness of the tongue, and dysphagia. Colic may be present as a result of progressive ileus. Foals are most often presented in recumbency and, when forced to stand, will show generalized muscle tremors because of severe weakness. Death occurs as a result of paralysis of the intercostal muscles and the diaphragm. Diagnosis of botulism is typically made by demonstration of the preformed toxin or C. botulinum spores in feed, serum, wounds, or gastrointestinal contents of affected horses.52 The mouse inoculation test commonly used in other species is not sensitive enough as the sole diagnostic test in the horse, because the amount of toxin necessary to kill a horse may not be enough to kill a mouse. Diagnosis is frequently made by the exclusion of other neurologic diseases. Results of hematologic analysis are often normal in these horses, with the exception of an elevated Pco2. Treatment consists of supportive care, ventilatory support, and administration of botulinum antitoxin. Aminoglycoside and tetracycline antibiotics should not be used because they can potentiate neuromuscular blockade. The antitoxin should be specific for the type of botulism involved, although the type is frequently not known. The most common type of botulism in North America is type B, although types C and A have been reported. A polyvalent antiserum is available, but the cost is prohibitive for most horse owners. Antitoxins are only effective against unbound toxin and must be given early in the course of the disease to be beneficial. The prognosis in all cases of botulism should be guarded. Vaccines against type B botulism are commercially available and are recommended on farms where the disease is endemic.
Trypanosomiasis
Trypanosoma evansi is a protozoal parasite that causes a chronic wasting syndrome in horses and numerous other species, including cattle and dogs. Less frequently, it can cause a severe encephalitis or myeloencephalitis with asymmetric leukoencephalomalacia, edema, demyelination, and lymphoplasmacytic perivascular cuffing.53 Neurologic signs reported include ataxia, blindness, nystagmus, head tilt, circling, head pressing, proprioceptive deficits, and hyperexcitability leading to obtundity and death.53,54 The use of subtherapeutic doses of antitrypanosomal drugs, such as diminazene aceturate, may clear the organism from peripheral tissues but appears to predispose the animal to developing CNS infection. Introduction of naïve animals into endemic areas may also account for the severity of signs seen with the neurologic disease. Serology or polymerase chain reaction (PCR) can be used for diagnosis. Once horses develop neurologic signs, the prognosis is grave.
Photic Head Shaking
No visual abnormalities are associated with photic head shaking.39,55 The underlying cause of head shaking in most horses is unknown. Proposed causes include iris cysts,39 guttural pouch disease, nasal and dental disease, middle ear disorders, and ear mite infestation (Trombicula autumnalis). A thorough examination should include endoscopy and radiography of the guttural pouches and upper airways and complete ophthalmic, otoscopic, and dental examinations.56 A more detailed examination should include observation of the horse during exercise and at rest with a fitted nasal mask and tinted contact lenses, as well as during anesthesia of the infraorbital and posterior ethmoidal branches of the trigeminal nerve.57 Medical management may include administration of cyproheptadine, an antiserotonergic drug thought to decrease neuropathic pain, with or without concurrent administration of carbamazepine, a drug related structurally to the tricyclic antidepressants, which is thought to reduce nerve impulses in the trigeminal nerve and has been used frequently for the treatment of trigeminal neuropathy in humans.57,58 Additional procedures that have been recommended include posterior ethmoid nerve sclerosis and infraorbital neurectomies, although significant adverse effects may be associated with these procedures.56,57
Ischemic Optic Neuropathy
Epistaxis caused by guttural pouch mycosis can be treated with arterial occlusion of the internal carotid, external carotid, and greater palatine arteries. This treatment can result in sudden irreversible blindness of the eye on the affected side. Clinical lesions include congestion of the optic nerve head and involvement of the nerve fiber layer (see Chapter 9).59 Severe blood loss can result in bilateral blindness caused by an unknown mechanism.60
Intracranial Neoplasia
Intracranial neoplasia is rare in the horse. Clinical signs usually relate to the size and location of the tumor and whether metastasis has occurred. Exophthalmos, ocular discharge, and facial deformity may be observed with masses involving the sinuses or nasal passages.61–65 Unilateral blindness, ventrolateral strabismus, and a lack of physiologic nystagmus have been described in a horse with intracranial neoplasia that involved the retrobulbar space.66 These lesions were thought to be due to involvement of CNs II, III, and IV and possibly CN VI through compression by a space-occupying mass or metastasis of the tumor to involve the peripheral nerves. An immune-mediated paraneoplastic peripheral neuropathy may also have contributed to the dysfunction of these peripheral nerves.66 CSF cytology, radiography, endoscopy of the nasal passages, and CT or MRI can all be used to assist in making the diagnosis.66 A prognosis should be determined according to the extent and nature of the lesion and the possibility for surgical removal or adjunctive therapy.
Polyneuritis Equi
Polyneuritis equi is a chronic disease characterized initially by hyperesthesia, followed by progressive hypoesthesia and subsequent paralysis of regions innervated by the corresponding nerve roots. Common areas affected include the tail; anus and perineal area; bladder; and CNs V, VII, and VIII, although other cranial nerves may be affected.67 CNs VII and VIII are more commonly affected than CN V.38 Facial nerve paralysis and decreased tear production can occur with secondary corneal disease.68 Colic, as a result of fecal retention and bladder distension, is a common clinical sign. The cause is unknown, but a viral or immune-mediated etiology has been proposed. This is supported by the presence of antibodies to P2 myelin protein that are found in some horses with the disease.69 This antibody has also been found in horses with equine herpesvirus 1 (EHV-1) and adenovirus infections, however, and should only be considered as a supportive test. Other diagnostic tests should include a complete blood count, which often shows signs of chronic inflammation (mature neutrophilia with hyperfibrinogenemia), and CSF cytology, which may be normal or may show high white blood cell counts or protein levels.69,70 The definitive diagnosis is often made at necropsy. Histopathologic examination may reveal inflammation and infiltration of the extradural or intradural nerve roots.71 Treatment with steroids may improve clinical signs in the early stages of the disease, but the lesions are progressive, and euthanasia is often required.
Viral Diseases
Equine Viral Arteritis
Equine viral arteritis is caused by a togavirus and is spread by aerosolization and inhalation or through sexual contact. Carrier stallions are considered responsible for maintaining the disease in the population and may shed the virus in their semen for months to years.72 Outbreaks have occurred in New Mexico (2006) and France (2007) that highlight the potential for the quick dissemination of the disease among breeding farms.73 There are two major syndromes associated with equine viral arteritis in the horse: a respiratory syndrome and an abortion syndrome. Many cases are asymptomatic, but clinical signs may include pyrexia, upper respiratory tract disease, severe edema, and abortion 3 to 8 weeks after infection with the virus.74 The disease is often fatal in neonatal foals. Ocular signs include serous to mucoid ocular discharge, supraorbital and periorbital edema, conjunctivitis, corneal opacity, and photophobia (Box 13-5). The pathophysiology involves a necrotizing vasculitis, mainly of the small arteries in the muscle tissue of affected animals.75 Diagnosis is based on seroconversion, virus isolation, or results of PCR.72 Leukopenia with a lymphopenia is a consistent finding on hematologic analysis. There is no specific treatment. Prevention involves vaccination of mares and prepubertal stallions and routine testing of breeding stallions. Freezing of the semen will not deactivate the virus, and both freshly collected and frozen semen are considered infective.75
Equine Infectious Anemia
Equine infectious anemia (EIA) is caused by a nononcogenic retrovirus that produces an immune-mediated hemolytic anemia in affected horses.76 The disease originally presents as an acute syndrome characterized by fever, anorexia, depression, and petechial hemorrhages caused by thrombocytopenia. Thrombocytopenia can have associated ocular lesions, including conjunctival and intraocular hemorrhages. Choroiditis has also been documented with EIA.77 Anemia develops in the subacute to chronic stage, and horses with EIA may have a recrudescence of the disease. Chronic carriers have also been described, and these horses are clinically healthy and hematologically normal. Diagnosis is most frequently made by the Coggins test; the result is positive as early as 10 days after infection and remains positive as a result of chronic antigenic stimulation from the virus.76 No treatment or vaccine is available for EIA, and infected horses are considered contagious. The disease must be reported, and euthanasia is recommended.
Rabies
Rabies is caused by a neurotropic rhabdovirus that affects most warm-blooded mammals. The disease is rapidly progressive after a variable incubation period, and it is invariably fatal. Early clinical signs are nonspecific and often include muzzle fasciculations, behavioral changes, hindlimb lameness or paresis, colic, and pharyngeal paralysis.78,79 Recumbency, convulsions, head pressing, and circling may occur as terminal events.23 Ocular signs are rare and include blindness, prolapse of the third eyelid, nystagmus, and strabismus, most likely caused by diffuse cerebrocortical edema and hemorrhage.77–79 The retinal ganglion cells may be susceptible to infection.50 There is no treatment available for rabid animals, and euthanasia is recommended. Appropriate caution should be taken with all animals with neurologic signs, especially those horses that have not been vaccinated for rabies. The most reliable diagnostic test is the fluorescent antibody test performed on brain tissue.78
African Horse Sickness
African horse sickness is not present in North America but is endemic in sub-Saharan Africa. The virus is a noncontagious, arthropod-borne orbivirus that affects all Equidae; however, mules, donkeys, and zebras are less susceptible than horses.80 Four forms of the disease are recognized: the pulmonary or peracute form, the cardiac or subacute form, the mixed form, and a milder horse sickness fever.81 The mixed form is the one most commonly diagnosed and has clinical signs from both the pulmonary and cardiac forms of the disease, which may include pyrexia; tachypnea; sweating; cough; mucosal congestion; pleural effusion; and pulmonary, interfascial, and subcutaneous edema.80 Bulging of the supraorbital fossae (Fig. 13-10) occurs in 10% to 20% of cases, but it is a hallmark sign considered diagnostic for the disease (Box 13-6). This is likely due to retrobulbar edema and increased vascular pressure. Another possible ocular sign is marked chemosis (conjunctival edema).82 The mortality rate is between 50% and 95%. Diagnosis is based on virus isolation or neutralization and serology. Prevention involves quarantine of affected animals and vaccination of animals in endemic areas.83
Equine Herpesvirus
Equine herpesvirus 1 (EHV-1) is a common cause of upper respiratory tract infection, abortion, and neurologic disease in horses. Respiratory signs are more common in young horses and show horses and include fever, cough, and a mucoid nasal discharge that may progress to mucopurulent discharge with secondary bacterial infections. Abortions tend to occur after the seventh month of gestation and are sudden, without premonitory signs.84 Stillbirths may also occur, and foals born alive are often weak, have severe interstitial pneumonia, and typically die within the first few days of life. The neurologic form of EHV-1 frequently presents with hindlimb ataxia, decreased tail and anal tone, and fecal and urinary retention. Signs may progress to the forelimbs, and horses may eventually become recumbent, which indicates a poor prognosis.
Ocular signs associated with EHV-1 or EHV-4 include hyperemia of the conjunctiva or sclera. Experimental infection of a foal with EHV-1 led to signs of severe visual dysfunction and chorioretinitis 28 days after exposure.85 Ophthalmic exam revealed severe degeneration of the neurosensory retina, retinal pigmented epithelium, and choroidal layers, with EHV-1 DNA present in the ocular tissues. This lesion has not been reported in naturally occurring infection. Infection with the neuropathic strain of EHV-1 may result in diffuse cerebral dysfunction and cranial nerve involvement.
Treatment with the antiviral drug valacyclovir has been evaluated, but its benefits are questionable, and it remains cost prohibitive at this time.86 Supportive care including antiinflammatory treatments, stall rest, and adequate nursing for horses with the neurologic form of the disease is recommended. Commercial vaccines against EHV-1 and EHV-4 are available, although protection is only short lived, and the vaccines may not protect against the neurologic form of the disease.
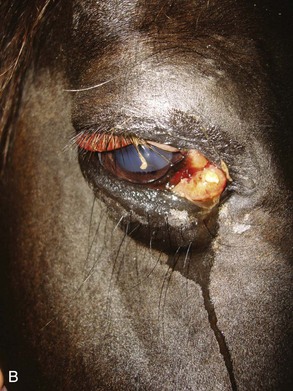
EHV-2 is a cytomegalovirus not known to cause disease by itself but considered important in the pathogenesis of other diseases through immunosuppression or possible transactivation of EHV-1.87–89 The virus has been detected in pulmonary macrophages of up to 90% of horses with chronic pulmonary disease.90 There are numerous reports of ocular signs associated with EHV-2, including serous to mucopurulent ocular discharge, conjunctival hyperemia and chemosis, superficial dendritic-type corneal lesions, which may be linear or punctate, and corneal edema and vascularization (Fig. 13-11).7,91 Response to topical antiviral medications is often favorable.92,93 Topical application of interferon and oral administration of l-lysine may be helpful (see Chapter 5 for more information).
Adenovirus
The most frequent manifestation of adenovirus in the horse is as a lower respiratory tract pathogen in foals with severe combined immunodeficiency.94,95 The virus has also been co-isolated from foals with diarrhea caused by rotavirus.96 It is not considered a pathogen in nonimmunocompromised animals. Bronchopneumonia is accompanied by mucopurulent nasal and ocular discharge. Conjunctival epithelial cells are histologically necrotic with intranuclear inclusions, and a neutrophilic infiltrate is present in the uveal vasculature, which is consistent with panuveitis.7,97
Equine Influenza
Clinical signs of equine influenza virus are virtually indistinguishable from those of upper respiratory tract disease caused by EHV-1 and EHV-4. Fever, cough, and a serous or mucoid nasal and ocular discharge are often present along with conjunctival hyperemia (see Box 13-5).7 The disease most often affects young horses, and there is usually a history of travel or introduction of a new horse into the herd. The virus spreads extremely rapidly, with an onset of fever within 2 to 3 days, which may last for up to 10 days, and a harsh, dry cough, which may persist for 2 to 3 weeks.98 Treatment consists of supportive therapy, administration of nonsteroidal antiinflammatory drugs (NSAIDs), and stall rest for several weeks after resolution of clinical signs. Immunity after natural infection may last for more than 1 year; however, immunity after vaccination usually only lasts for 2 to 3 months because of antigenic drift in the virus.98,99 The modified live intranasal vaccine may provide extended immunity (>6 months) and has proven effective in challenge studies.100
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree