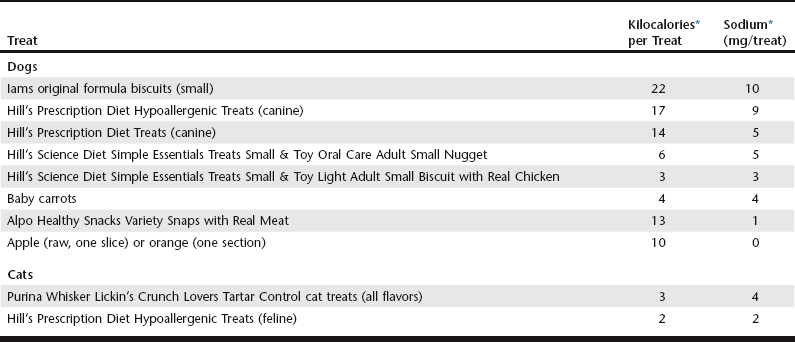
Chapter 168 A single diet does not work for all animals with heart disease, and dietary modifications need to be individualized. Patients with heart disease vary in their clinical signs, laboratory parameters, and food preferences, all of which affect diet selection. For example, more severe sodium restriction would be required for a cat with congestive heart failure (CHF) than for a cat with asymptomatic heart disease. Animals with heart disease may be hyperkalemic, hypokalemic, or normokalemic, which influences the choice of diet. Concurrent diseases also alter diet choice and are present in many animals with heart disease (61% of dogs and 56% of cats) (Freeman et al, 2003; Torin et al, 2007). In addition to the pet food selected, the owner also must receive careful instructions on treats and table food. In some cases animals may be eating an ideal pet food but are obtaining large amounts of sodium from treats. Over 90% of dogs and 30% of cats with heart disease received treats (Freeman et al, 2003; Torin et al, 2007). Examples of appropriate treats are given in Table 168-1. Including all sources of dietary sodium in the overall diet plan is important to achieve success with nutritional modifications. Although a diet must be selected based on desired nutritional properties and palatability, it also is important to devise an overall dietary plan that includes an effective method for administering medications. Most dog owners (57%) and many cat owners (34%) use food for medication administration, and the most commonly used foods are high in sodium (e.g., cheese, hot dogs, lunch meats) (Freeman et al, 2003; Torin et al, 2007). Therefore examples of appropriate methods for administering medications should be provided (Box 168-1). One of the earliest and major compensatory responses in heart disease is activation of the RAA system, and sodium restriction can further elevate renin, angiotensin, and aldosterone concentrations. Thus severe sodium restriction in animals with early heart disease theoretically could be detrimental by triggering early and excessive activation of the RAA system. I recommend only mild sodium restriction in asymptomatic heart disease (<100 mg/100 kcal for animals with International Small Animal Cardiac Health Council [ISACHC] class 1a or 1b heart disease; see Chapter 176). However, this is also an opportune time to begin talking to the owner about the animal’s overall dietary patterns (e.g., the pet’s food, treats, table food, and methods of administering medications), since it is generally much easier to institute dietary modifications when the animal is asymptomatic. Finally, there is the potential for benefit from nutritional modification in the asymptomatic animal. One study compared a moderately reduced-sodium cardiac diet that was enriched with omega-3 fatty acids, antioxidants, arginine, taurine, and carnitine with a placebo diet in dogs with asymptomatic chronic valvular disease (CVD) (Freeman et al, 2006). The cardiac diet increased circulating levels of key nutrients (e.g., antioxidants, omega-3 fatty acids) and also reduced cardiac size. This reduction in cardiac size did not appear to be an effect of sodium restriction. In addition, one retrospective study showed a significantly longer survival time in dogs with cardiac disease that were receiving omega-3 fatty acid supplementation (Slupe et al, 2008). Future prospective studies are needed to confirm such findings and to provide a better understanding of the role of nutritional modification in this early stage of disease. Unlike in the healthy animal or the asymptomatic person, dog, or cat with cardiac disease, obesity actually may be associated with a protective effect once CHF is present; this is known as the obesity paradox (Finn et al, 2010; Slupe et al, 2008). Although there are a number of hypothesized reasons for the obesity paradox, the benefit of obesity in CHF likely is due more to a lack of cachexia than to the obesity per se, given the adverse effects associated with cachexia.
Nutritional Management of Heart Disease
General Nutritional Issues for Animals with Heart Disease
Nutritional Modifications Based on Severity of Disease
Asymptomatic Heart Disease
Mild to Moderate Congestive Heart Failure
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Nutritional Management of Heart Disease
Only gold members can continue reading. Log In or Register to continue