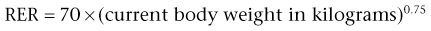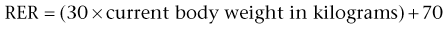Chapter 7 Indicators of malnutrition in animals that have been proposed include unintentional weight loss (typically greater than 10% of body weight), poor hair coat quality, muscle wasting, signs of inadequate wound healing, and hypoalbuminemia. However, these abnormalities are not specific to malnutrition and often occur as late complications of a variety of systemic diseases. A greater emphasis is placed on evaluating overall body condition rather than simply noting body weight. Body condition scores (BCSs) have been shown to be reproducible, reliable, and clinically useful in nutritional assessment. Fluid shifts may significantly impact body weight, but BCSs are not affected by fluid shifts and therefore are helpful in assessing critically ill animals. More recently, lean muscle loss has also been evaluated in cats and this may become a component of nutritional assessment in small animals (Michel et al, 2011). Alternatively, for animals weighing between 3 and 25 kg, the following may be used: Lipid emulsions are the calorically dense component of PN and a source of essential fatty acids. Lipid emulsions are isotonic and available in 10% to 30% solutions (e.g., Intralipid, Liposyn III). These commercially available lipid emulsions are made primarily of soybean and safflower oil and provide predominantly long-chain polyunsaturated fatty acids, including linoleic, oleic, palmitic, and stearic acids. The emulsified fat particles are comparable in size to chylomicrons and are removed from the circulation via the action of peripheral lipoprotein lipase. Side effects attributed to lipid emulsions include liver dysfunction and immune suppression. Newer lipid emulsions with fewer side effects have been developed, and one such product composed of soybean oil, medium chain triglycerides, olive oil, and fish oil (i.e., SMOF lipid) is available in Europe and may become more widely distributed in the future (Goulet et al, 2010). There is a persistent misconception regarding the use of lipids in cases of pancreatitis. Although hypertriglyceridemia may be a risk factor for pancreatitis, infusions of lipids have not been shown to increase pancreatic secretion or worsen pancreatitis and therefore are considered safe. However, the one exception is in cases in which serum triglyceride concentrations are severely increased, indicating a clear failure of triglyceride clearance. Although specific data regarding the maximal safe level of lipid administration in veterinary patients are not available, it would seem prudent to maintain normal serum triglyceride concentrations in patients receiving PN. Another concern surrounding the use of lipids in PN is their purported immunosuppressive effects via impairment of the reticuloendothelial system, particularly in PN solutions containing a high percentage of lipids. Despite in vitro evidence supporting the notion that lipid infusions can also suppress neutrophil and lymphocyte function, studies have not yet correlated lipid use and increased rates of infectious complications.
Nutrition in Critical Care
Nutritional Assessment
Calculating Nutritional Requirements
Parenteral Nutritional Support
Nutrition in Critical Care
Only gold members can continue reading. Log In or Register to continue

Full access? Get Clinical Tree