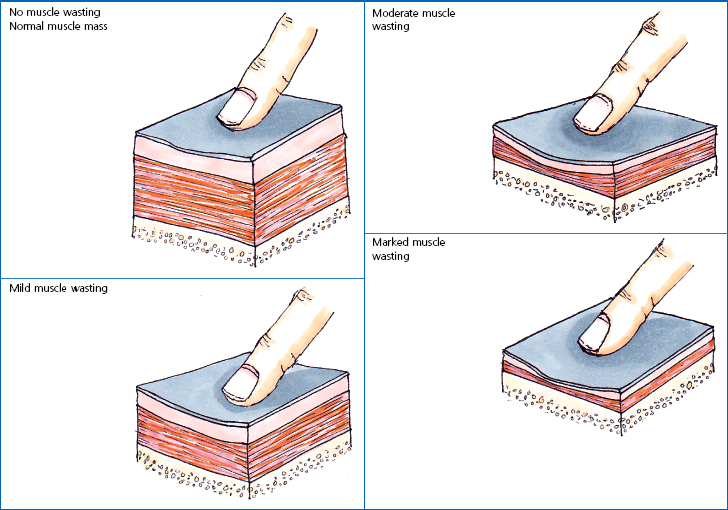
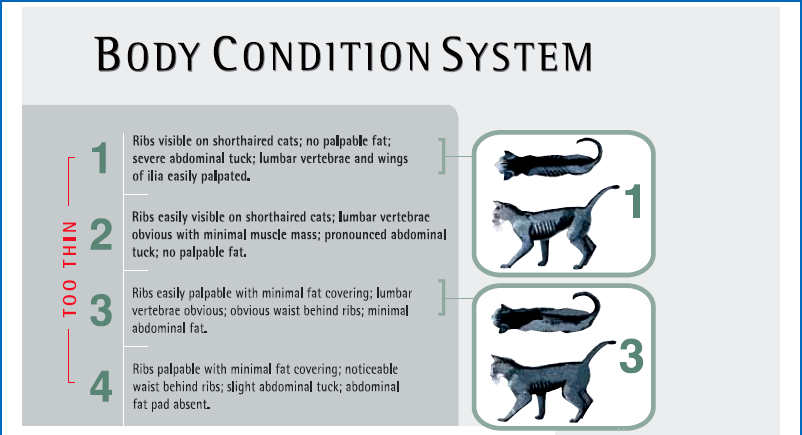
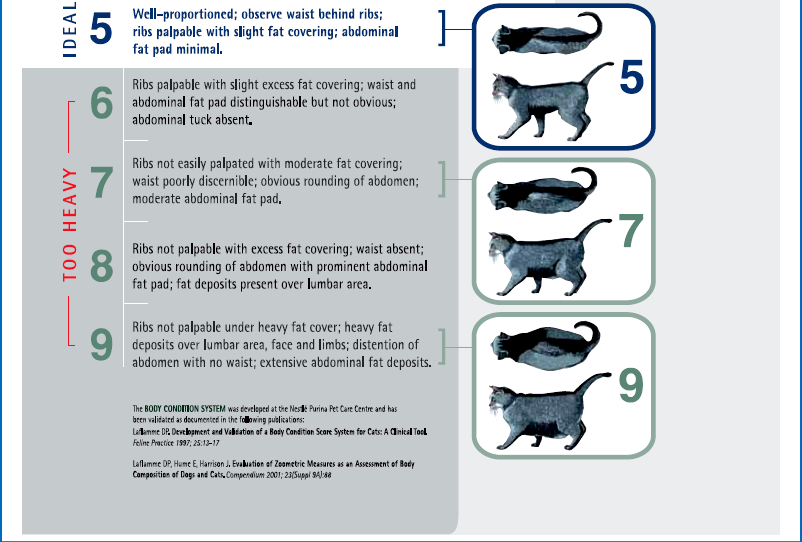
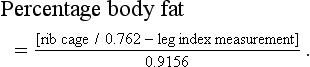Chapter 6 Preoperative nutritional status in people predicts the occurrence of postoperative complications and the severity of the inflammatory response.1–4 Deterioration of nutritional status in patients undergoing major surgery is associated with poor wound healing and decreased immune function.4 Other consequences of malnutrition include fatigue, decreased thermoregulation, respiratory, pancreatic, gastrointestinal, mental, endocrine and cardiovascular functions, and impaired muscle strength.5 Morbidity and mortality rates are increased in malnourished patients. The postoperative mortality rate in human cancer patients approaches 23% for the malnourished but is only 4% in those in better nutritional condition, unrelated to differences in disease or procedure.6 Nutritional support in elderly patients reduces the mortality rate from 18.6% to 8.6% at six months.7 Similarly, emaciated cats are significantly more likely to die within a year than those of optimal body condition.8 Conversely, obesity has been cited as increasing the potential anesthetic and surgical risks in people and dogs,9,10 though this is less well documented in cats. Obesity influences the dosing of pre-anesthetic and anesthetic medications, as dosing for actual weight rather than for lean body weight may result in over-dosing. With repeated dosing of some intravenous drugs there is saturation of lean body tissue and drug distribution to fat, which may result in prolonged recovery times.11 Further, obesity can make monitoring by auscultation during anesthesia more difficult due to the muffling of heart and lung sounds.12 Nutritional management starts with assessment of the nutritional status, including dietary history. Dietary history should include the cat’s appetite, the food brand name, flavor and type, amount fed, number of meals, and any treats, snacks, or foods given to administer medications. If present, the duration of anorexia or decreased appetite should be noted. People give unreliable estimates of their own food intake,13 but the reliability of an owner’s assessment of their pet’s food intake is unknown. Any history of weight loss or gain should be noted, though assessing weight change can be difficult if the animal’s previous weight is unknown. Ascites may make weight loss more difficult to assess. Impairment of organ function occurs with starvation before significant physical changes are observed, and nutritional deprivation is especially likely to be overlooked in the obese hospitalized patient.6 Initiation of nutritional support is indicated in patients meeting any of these criteria: acute weight loss of >5%, chronic weight loss of >10%, poor body condition, anorexia for more than three days, decreased protein intake or malabsorption, weakness, or non-healing wounds (Box 6-1). Many assessment protocols for humans use the body mass index (BMI), which is weight (kg)/height(m)2, and anthropometric measurements such as midarm muscle circumference.6 A feline version of BMI has been developed and validated by dual energy X-ray absorptiometry (DEXA) for healthy cats weighing between 3 kg and 9 kg.14 The feline BMI uses the rib cage circumference in cm and the leg index measurement (LIM), which is the hind leg length in cm from the middle of the patella to the dorsal tip of the calcaneal process. The amount of body fat is estimated using the equation in Box 6-2. This calculation has not been validated in ill cats, and feline BMI is not widely used in clinical practice. Body condition scores (BCS) provide a semi-quantitative method for evaluating fat and lean body tissue percentages.15,16 Scoring systems have been developed using systems of 1 to 5, 6, or 9 points, with lower numbers representing thinner cats and higher numbers indicating fatter animals. A feline BCS system using a 9-point scale has shown good correlation (0.91) with percentage of body fat as determined by DEXA.16 This BCS system, using pictures and descriptions, shows good repeatability within and between observers. A score of five out of nine is optimal (Table 6-1). Table 6-1 Body Condition Scores in cats. Image and intellectual property items are produced with the kind permission of Société des Produits Nestlé S.A. The BCS systems do not take into account muscle wasting, and many ill cats have muscle loss disproportionate to fat loss due to the metabolic changes of stress. Consequently, a 4-point feline muscle mass scoring (MSS) system has been developed based on palpation of muscles over the skull, scapulae, spine, and pelvis17 (Table 6-2). Animals with no muscle wasting are scored as a 3, mild wasting is 2, moderate wasting is 1, and a 0 score represents severe wasting. There was a fair correlation between the feline MMS system and cats’ lean body mass as determined by DEXA.17 Multi-frequency bioelectrical impedance has been used experimentally to assess body composition in healthy cats,18 but is not used in the clinical setting. Disturbances of water and electrolyte metabolism in the ill patient limit the usefulness of this technique in the hospital.6 In research settings, DEXA is used for determination of body composition. It has good accuracy but requires anesthesia and is not practical for most patients.19 Nutritional assessment in humans has included measurements of serum albumin and other proteins, including acute phase reactants. These parameters are affected by factors other than nutrition, including metabolic changes of illness, hydration, and vascular permeability. Serum albumin does not correlate well with other nutritional assessments or with starvation in people,6,20 although in cats admitted to a referral practice there was a positive correlation of serum albumin with body condition score.21 This was likely due to decreases in serum albumin and BCS both reflecting the underlying disease.20,21 Serum insulin-like growth factor (IGF)-I concentrations are reduced by short-term dietary restriction and restored by re-feeding in cats,22 but this parameter is not widely available for daily use in practice. Fasting has been recommended prior to general anesthesia to minimize gastric content volume and thereby decrease the risk of regurgitation and aspiration. Gastroesophageal reflux during general anesthesia is considered to be a common cause of esophagitis, and can cause esophageal strictures. The incidence of gastroesophageal reflux during anesthesia in dogs has been reported as 16.3%.23 In another canine study, the rate of esophageal stricture caused by gastroesophageal reflux during anesthesia was 0.13%, and 30% of these cases died.24 Studies in cats are not available. The traditional practice in veterinary medicine has been to starve patients overnight or for at least six to 12 hours prior to anesthesia. However, canine studies have shown that prolonged fasting neither diminishes gastric volume nor decreases gastric acidity.25 An increased duration of fasting is actually associated with an increased incidence of reflux in dogs. None of 30 dogs fasted for two to four hours refluxed during anesthesia, while 13.3% of the ones fasted for 12 to 18 hours had an episode of reflux.23 In another study, none of 31 dogs fasted for three hours refluxed, while 16.3% of those fasted for ten hours had an episode. Dogs fasted for three hours after a canned food meal did not have a significantly increased gastric volume compared to those dogs fasted for 20 hours. Further, gastric acidity was reduced in the dogs with a three hour fast compared to those with a 20 hour fast.26 Morphine also decreases the lower esophageal pressure; its use in combination with acetylpromazine (acepromazine) prior to anesthesia increased the incidence of gastroesophageal reflux in dogs from 27% to 60%.27 Further, in humans, providing oral fluids and carbohydrates up to two hours before surgery attenuates postoperative insulin resistance. As dietary fat slows the rate of gastric emptying, feeding a small, low fat meal may be the best option.28 Recent recommendations for otherwise healthy dogs undergoing elective procedures are to allow water up to two hours prior to induction, and a light meal three to four hours before induction.29 While no feline studies are available to provide specific protocols, similar recommendations are reasonable. No studies provide good evidence for the most appropriate preoperative fasting times for kittens. Similar to current recommendation for adult animals, many clinicians provide a small morning feed three to four hours prior to anesthesia to decrease the risk of hypoglycemia.30 Checking blood glucose concentration at induction and intermittently during a procedure and treating as needed may be recommended for very young kittens, especially for longer procedures. Where possible, surgery should be delayed until the cat’s diabetic state is stable and reasonably well controlled with insulin. While surgery itself is not a greater risk for a well-controlled diabetic patient than for a healthy animal, the preoperative fast and the anesthetic drugs may cause problems, as can stresses which cause release of counter-regulatory (diabetogenic) hormones promoting insulin resistance and ketogenesis.31 Even short hypoglycemic episodes may cause brain damage and hyperglycemia can cause an osmotic diuresis.11 Various different options are available for diabetic cats (Chapter 2); ultimately, careful monitoring of blood glucose is essential in all cases. One option is to provide food and insulin in the morning as normal and anesthetize in the afternoon. Otherwise, insulin should be administered in the morning, but if the cat will not be fed, the insulin dose should be adjusted31 as recommended in Table 6-3. Table 6-3 Adjusting dextrose and insulin doses prior to anesthesia
Nutrition for the surgical patient
Preoperative assessment
Body assessment
How long to starve before surgery?
Adult cats
Kittens
Diabetic cats
Morning blood glucose (mmol/L) (before insulin)
2.5–3% dextrose infusion
Insulin (percentage of normal dose)
<5.5
Yes
None
5.5–11
Yes
25%
11
Not until glucose is ≤8 mmol/L
50%
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine