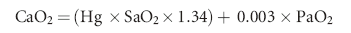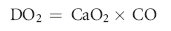Chapter 34 Nonrespiratory Look-Alikes
pH and pCO2 RECEPTOR ACTIVATION
The most common acid-base abnormality in small animals is metabolic acidosis.4 Metabolic acidosis is characterized by an increased hydrogen ion concentration, decreased pH, and decreased bicarbonate ion (HCO3−) concentration (see Chapter 59, Acid-Base Disturbances).5 Metabolic acidoses most often result from a loss of bicarbonate-rich fluid from the body, increased hydrogen ion production, or decreased renal hydrogen ion excretion. In small animal medicine, the more common processes that cause metabolic acidoses are diabetic ketoacidosis, diarrhea-induced hyperchloremic acidosis, lactic acidosis, and uremic acidosis.5 The normal compensatory mechanism for metabolic acidosis is to expel additional carbon dioxide via hyperventilation, as evidenced by a decrease in the partial pressure of carbon dioxide in the peripheral arterial blood (PaCO2). This compensatory mechanism is initiated as a result of the increased number of hydrogen ions stimulating peripheral and central chemoreceptors, which in turn increases alveolar ventilation (hyperventilation).5
pO2 RECEPTOR ACTIVATION
Recall that the formula to determine arterial oxygen content (CaO2) is:
and for oxygen delivery (DO2) is:
where Hg is the hemoglobin concentration (g/dl), SaO2 is the percent oxyhemoglobin saturation of arterial blood, PaO2 is the partial pressure of oxygen in the arterial blood (mm Hg), and CO is cardiac output (dl/min).7
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree