Neonatology and Neonatal Disorders
The Abnormal Neonate
In South America, 20% to 80% of crias do not survive to weaning. Deaths occur for a variety of reasons, many of which have not been cataloged. In North American populations, the death rate is lower, but figures are not available. Well-managed farms should aim to lose fewer than 5% of their crias in a year. In general, crias suffer from the same maladies as other neonatal livestock. Dysmaturity, prematurity, dystocia, sepsis, and starvation are common problems and should be handled in the same way as in other species. In contrast, crias appear to have higher rates of certain problems such as those related to congenital malformations.
Abnormal neonates may initially show few overt signs of disease or may show signs that are vague. Given how rapidly sick neonates can deteriorate, even these small abnormalities may indicate the need for aggressive action. Abnormalities are best detected through a thorough postnatal checkup, routine, consistent monitoring of progress according to the timetable in Chapter 25, generally observing activities, including nursing, elimination, and physical activity, and charting changes in size and body weight. Even small changes such as failure to gain weight over a 24-hour period while all else appears normal could be significant, and any delay in investigating these changes and initiating treatment could result in more advanced clinical disease. Interventions may be anything from modest management changes to intensive medical care. Identifying the neonates that require interventions and determining the level are matters of experience and good diagnostic evaluation.
Slow-starting crias may require little more than simple, but immediate, intervention. The most vital events of the first few hours of life are breathing, standing, and nursing. Breathing is the most urgent. Crias with overt dyspnea, including open-mouthed breathing or stertorous respiration, should be maintained in sternal recumbency or assisted to stand. The airway should be assessed for obstruction. The external nares should be cleared. A bulb syringe may be used to blow mucus back into the oropharynx, and a soft rubber urinary catheter may be inserted past the level of the eye in each nares for a quick assessment of choanal patency. Rubbing the thorax and dorsum also may stimulate breathing efforts. Breathing function may be assessed by arterial blood gas (ABG) analysis and thoracic radiography, and endoscopy or imaging studies may be used to assess airway patency (see Chapter 37). If hypoxemia is a concern, the cria may be placed on supplemental oxygen, and if ventilation is an issue, the cria may be intubated for mechanical ventilation. Face masks used for small animal patients may be tolerated in the short term but are hard to maintain. Nasal prongs used in human hospitals work well for crias and are self-retaining. Insufflation tubes or catheters placed in a nostril are also acceptable. When possible, supplemental oxygen should be warmed and humidified. Assessment of breathing function may be indicated even in the absence of dyspnea. Hypoxemia and hypercapnea are both important causes of neonatal obtundation, so supplemental oxygen or mechanical ventilation should be considered in any dull cria. Moribund crias are at risk of corneal ulcers because of reduced blinking reflexes. Ocular lubricants and common sense management to avoid eye trauma may be indicated.
During the period of initial assessment, after airway and breathing issues are sorted out, the cria should be examined for evidence of prematurity and external defects. Signs of prematurity are as follows: overt external defects such as facial malformations, cleft palate, limb abnormalities, a bleeding umbilicus or umbilical hernia, patent urachus, malformations of the external genitalia (Figure 42-1), and absence or atresia of the anus. Mucous membranes and sclerae should be examined for cyanosis, injection, vascularity and refill times, and the anterior chamber of the eye should be examined for hypopyon. Normal vital parameters and issues with cardiac auscultation have been discussed earlier in this text. It is important to realize that sepsis leads to hypothermia more commonly than fever. Pulmonary auscultation is the same as in other species. Some degree of “squeakiness” is normal in the immediate postpartum period, but this should resolve within an hour. Neurologic examination is difficult to interpret in the immediate postpartum period or in severely obtunded crias but may be useful in older crias.
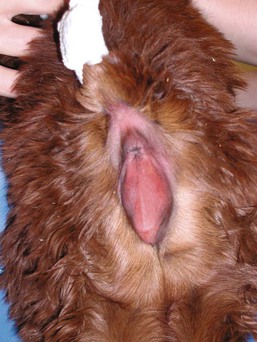
Figure 42-1 Posterior view of a female cria with an imperforate vulva. Note the complete absence of an opening in the vulva.
Breathing and standing are usually prerequisites to nursing, but in their absence, some form of intervention to provide nutrition and immunoglobulin (Ig) is required. If the dam has colostrum, the cria should preferably be assisted to nurse or the dam milked out so that the cria can be fed via a bottle or tube. Dams may be restrained against stall walls to allow recumbent crias to nurse (personal communication, Dr. D. Mora). Colostrum or milk may be given via a tube, pan, or bottle, and intravenous (IV) nutrition or plasma transfusion may be initiated. If the dam’s colostrum is not available, camelid or ruminant colostrum may be administered for the first 12 to 24 hours. Ruminant colostrum should be from Johne’s free herds or flocks, if possible. Bovine colostrum has also been associated with immune-mediated hemolysis in rare cases.
If colostrum is not available within the first few hours after birth, administering milk or a milk replacer is likely to be beneficial in providing fluid volume, nutrition, and the impetus for gut closure. Warmed goat or cow milk or milk replacer may be used, and an initial volume equal to approximately 3.5% of body weight appears adequate. If milk is administered in the absence of colostrum, plasma transfusion should be strongly considered. Although it has not yet been studied scientifically, some think that camelid milk has metabolic effects beyond simple nutrition, so any cria fed milk or milk replacer from another species should be observed carefully for changes in attitude or activity that could reflect the onset of a hyperglycemic disorder (see Chapter 41, “Hyperosmolar Disorder”). It is also important to remember that antibody and nutrient absorption may be affected by tissue function and oxygenation; hypoxic or otherwise compromised crias may require plasma transfusion or parenteral nutrition in spite of adequate colostral or milk ingestion or administration.
Clinical signs of dysmaturity are the same as those in other species and include obtundation, low birth weight, unerupted incisors, floppy ears or ears that are bent backwards (Figure 42-2), poor or absent suckle reflex, and silky haircoats. Tendon laxity (see later in this chapter and in Chapter 58)may result in overextension of the carpal and fetlock joints, so crias may be seen walking on their fetlocks (Figure 42-3). Crias may also have a rubbery covering on the nails of the feet and a thicker epidermal membrane that persists for longer than normal. The central incisors normally erupt 2 to 3 weeks prior to birth, so unerupted incisors suggest that a cria is underdeveloped at least to this extent. Dymature crias tend to be weak and may have difficulty standing or holding their heads up to nurse because of poor muscle development. Furthermore, dysmature crias may have incomplete structural and functional maturation of vital body systems, and this leaves them susceptible to a range of potential neonatal complications. These crias are at risk for hypothermia because their thermoregulatory control is poor, and they are prone to hyperglycemia or hypoglycemia. Respiratory system immaturity may result in poor lung expansion from lack of pulmonary surfactant, and this also reduces oxygen transfer across the alveoli. Poor oxygen diffusion together with bradypnea results in hypoxia. It is unknown when surfactant is produced in the lungs of camelids, but those with unerupted incisors are especially at risk. The intestinal mucosa may also be inadequately mature in premature crias, which results in inefficient absorption of colostral antibodies. More than half the dysmature crias presenting to one referral hospital had FPT.1
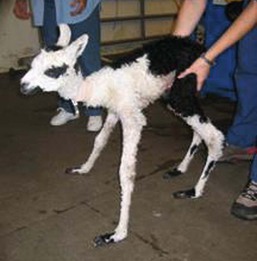
Figure 42-3 Tendon laxity in a newborn. Support wraps or splints are likely to be necessary in this case but may only be required for a few days.
Recognizing the clinical signs of dysmaturity is vital for newborn crias, as they may deteriorate rapidly. Early intervention is key to their survival. One report showed a 75% survival rate, and this was attributed to the fact that 87.5% of cases were presented within 24 hours of birth.1 Therapeutic measures may include oxygen administration, IV fluid administration, and provision of warmth. The contents and rate of administered fluids should be tailored to the specific needs and blood abnormalities; nutritional support should not be neglected. Additionally, aminophylline (2 mg/kg, subcutaneously [SQ], q4h for 24 hours, followed by q6h for the next 24 hours and q8h for the next 24 hours) may be used proactively or therapeutically when concerns about breathing exist. Lung surfactants have also seen some use.
The Critical Cria
Blood Evaluation
Hematologic evaluation is mainly helpful for diagnosing infection or anemia. With neonatal sepsis, gram-positive organisms often evoke neutrophilia with a left shift, whereas gram-negative organisms often acutely lead to neutropenia,2,3 which resolves over 3 to 5 days. Hyperfibrinogenemia, a left-shifted leukogram, and toxic changes in leukocytes both provide supportive evidence for sepsis but also may be absent in septic crias. The stress response, with its tendency to increase blood neutrophil counts, may mask neutropenia, so it is important to look for other evidence of stress (hyperglycemia, lymphopenia) and to mentally compensate for these: A normal neutrophil count in a stressed camelid, especially one with a left shift or toxic morphology of neutrophils may still be evidence of sepsis. Mycoplasma haemolamae infection has also been identified in newborns and may be identified on blood smear or by polymerase chain reaction (PCR).4
Blood biochemical abnormalities of particular importance include changes in blood glucose, electrolyte abnormalities, azotemia, metabolic acidosis with or without hyperlactemia or a high anion gap, hypoproteinemia (particularly hypoalbuminemia or hypogammaglobulinemia), evidence of fat mobilization, and increased activity of liver or muscle enzymes. It is a dangerous misperception that the majority of sick neonates need supplemental glucose. Only 13% of sick crias in one unpublished study had hypoglycemia, including only 11% of crias less than 24 hours old, but 14% had blood glucose concentrations greater than 200 milligrams per deciliter (mg/dL; 11 millimoles per liter [mmol/L]). It is, therefore, imperative to measure glucose before supplementing it. The most likely causes for hyperglycemia are stress and administration of glucose or a glycogenic agent, often compounded by insufficient water intake. Camelid milk may also play a role in suppressing hyperglycemia. The most likely causes for hypoglycemia are inadequate milk intake, potentially compounded by shivering or seizures, sepsis, or liver failure. If hypoglycemia is identified, it should be addressed promptly. The seizure threshold in crias appears to be just under 40 mg/dL (2.22 mmol/L) of glucose, so supplemental glucose is recommended for anything less than 70 mg/dL (3.9 mmol/L). Rapid infusion of 0.5 mL/kg of 50% dextrose, or preferably a slow infusion of 3 to 5 mL/kg of 10% dextrose over 5 to 10 minutes may be used.
High blood glucose concentrations become clinical through diuresis and dehydration of the brain and other tissues.5 Serum sodium concentration is a good indicator of the severity of dehydration from glucose diuresis: When sodium climbs to greater than 165 milliequivalents per liter (mEq/L), aggressive measures to replace fluid volume and decrease blood glucose are indicated. Very high glucose concentrations may be combated with insulin (regular insulin, 0.2 units/kg, IV, as often as hourly) or less aggressively with subcutaneous insulin. The combination of hyperglycemia and hypernatremia is termed hyperosmolar disorder. Its pathogenesis and treatment are discussed in Chapter 41. Hypernatremia and hyperchloremia are much more common in sick crias (roughly 14%) than hyponatremia or hypochloremia (roughly 1%), so salt loading should be avoided under most conditions.
Hypokalemia (38% of sick crias) caused by anorexia is relatively common and may be addressed by IV supplementation or milk feeding. Vigorous supplementation is rarely necessary. Azotemia was found in 14% of sick crias in one unpublished study. The minority of these had renal failure or congenital abnormalities; most were in hypovolemic or septic shock and responded well to fluids. Evidence of fat mobilization was not uncommon, and hepatic lipidosis was found in 12% of nonsurviving, live-born crias, with the youngest being 2 days old. These findings highlight the need for nutritional support, and that factors beyond pregnancy-related or lactation-related negative energy balance may lead to disorders of fat metabolism in camelids. Hyperlipemia, high blood concentrations of nonesterified fatty acids (NEFAs) or β-hydroxybutyrate (BHOB) or high activities on liver enzymes all suggest such a disorder might be developing. Diagnosis and treatment of disorders of energy metabolism is covered in Chapter 41.
Metabolic acidosis is more common. Simple bicarbonate loss is less common than in calves but is seen in some crias with diarrhea and others with apparent renal tubular acidosis. Acidosis without dehydration has also been described.6 The pathogenesis of this is not completely understood. Crias occasionally get lactic acidosis from grain overload or fermentation of milk, but hyperlactemia is more commonly the result of septic or hypovolemic shock. Ketoacidosis is also seen. Treatment of acidosis depends partially on the pathogenesis. With lactic acidosis or ketoacidosis, the primary strategies are to decrease production and increase elimination of the organic acid. Both these may often be achieved by rehydration. Ketoacidosis may also require the same treatment as that for hepatic lipidosis, and intestinal fermentative conditions may need to be treated in the same way as grain overload. If the blood pH is below 7.25, bicarbonate administration may be necessary as well. Sodium bicarbonate administration is also the cornerstone of treatment of bicarbonate-losing conditions, particularly when blood pH is less than 7.25. Multiplying the base deficit (or 24 mEq/L minus the observed bicarbonate value) by 0.5 by body weight in kilograms yields the total bicarbonate deficit. Half of this may be given within the first hour of fluids and the second half over the next 2 to 4 hours. Bicarbonate administration often temporarily corrects acidosis, but repeated monitoring is necessary to determine whether further doses should be given. Bicarbonate should also be avoided with respiratory acidosis, as hypercapnea may worsen.
Fluid Therapy
Plasma should be administered at 20 to 30 mL/kg. It is more effective than crystalloid fluids at expanding volume in sick neonates, since such crias often have increased endothelial permeability from inflammation. Plasma should be administered using a filtered administration set. Larger volumes (30 mL/kg) should be given over at least an hour. Faster administration may result in tachypnea, dyspnea, and evidence of central nervous system (CNS) disease. In one recent study, 30 mL/kg of camelid plasma was administered to clinically healthy alpaca crias with FPT over 90 minutes.7 This resulted in measurable plasma volume expansion, which appeared to be safe, and also a considerable improvement in arterial oxygen pressure. However, treated crias also showed a reduction in lung volume, and it was suggested that this may result in complications for those crias having preexisting cardiopulmonary compromise. Therefore, greater care would be advised in administering large-volume plasma transfusions to any cria with suspicion of cardiopulmonary issues or systemic disease. Monitoring changes in central venous pressures during a transfusion may be helpful in preventing such complications. Clinically, the observed response during a plasma transfusion may be quite rewarding.
By the time most crias present for evaluation, they often would benefit from IV fluids or other medications. Therefore, it is reasonable to place an IV catheter early in the evaluation (see Chapter 32) so that emergency treatment can be initiated while the examination is under way. Furthermore, blood samples can be collected from the catheter immediately after sterile placement for hematology, biochemistry, and culture, thereby reducing the number of venipuncture sites. Hematoma formation is even more common in crias than in adults, and each venipuncture increases the chance of introducing pathogenic bacteria into the bloodstream of the neonate.
Hypothermia
During pregnancy, the fetal temperature is 0.3°C to 0.5°C higher than that of the mother because of higher fetal metabolic rates. At birth, neonates are subject to a rapid drop in external temperature and need to rapidly increase heat production to keep warm. For this, they are highly dependent on nonshivering thermogenesis (NST).8 This process takes place in the mitochondria of brown adipose tissue and results in an uncoupling of fatty acid oxidation such that heat energy is produced instead of adenosine triphosphate (ATP). It is dependent on adequate oxygenation, making hypoxemic neonates more susceptible to the development of hypothermia. Additionally, premature neonates are likely to have inadequate reserves of brown adipose tissue, which develop late in gestation under the influence of NST inhibitors. Shivering generates additional heat, but the impact of this is small in neonates because of their small size and immature musculature. The presence of brown adipose tissue has not been specifically documented in camelids to date, so the extent of the role of NST in averting hypothermia in crias is uncertain. Thyroid hormones may also play a role in enhancing thermogenesis in the newborn. Neonatal llama crias have been shown to have very high blood thyroxine concentrations at birth, which decrease gradually over the first 90 days of life.9
Hypothermic neonates are depressed and lethargic and have reduced reflexes and poor ventilation. This, together with poor cardiac function, results in hypoxia, acidemia, and cardiac dysrhythmias.10 Hypothermic neonates are susceptible to FPT because of decreased nursing activity and possibly gut function. Hypoglycemia, although uncommon, may develop concurrently as hypothermic neonates deplete their energy reserves. Additionally, prolonged hypothermia may result in damage to the intestinal epithelium because of hypoxia, predisposing the neonate to clostridial overgrowth and pathogen invasion.11
Fever and Hyperthermia
The rectal temperature for normal crias is 37.8°C to 38.9°C (100°F–102°F).12,13 Therefore, hyperthermia in a neonate would be defined by a rectal temperature of 39°C (102.2°F) or greater. The most common cause for hyperthermia is fever related to infection. However, it is an inconsistent finding under those circumstances and should not be considered a prerequisite for a diagnosis of sepsis in camelids of any age. None of 21 crias with sepsis had a rectal temperature greater than 39°C (102.1°F) in one study, regardless of whether gram-positive or gram-negative bacteria were cultured from blood or tissue samples.2 In fact, 7 (33%) were actually hypothermic. In another retrospective study, only 2 of 6 crias with gram-negative sepsis were pyrexic.3 This compares well with findings in septicemic foals, in which only 30% of cases were pyrexic.13
The differential diagnoses for a high body temperature in neonatal llamas and alpacas include bacterial or viral infections, high environmental temperature, muscle tremors, or seizure activity. Bacterial infections are a likely sequel to failure of passive transfer. Muscle tremors may result from cerebral edema or ingestion of tremorgens. Seizures may result from a variety of CNS disorders (Box 42-1). In certain areas of the world, environmental temperatures may become extremely high and, especially when compounded with high humidity, may lead to hyperthermia. Neonates are particularly vulnerable if they are unable to seek suitable refuge, Furthermore, neonates that are suffering from underlying bacterial or viral infections are less able to thermoregulate and more susceptible to hyperthermia if unable to move to shade. High ambient temperatures may be focal as well, as in a stall with a heat lamp.
Fever is brought about by the production of pyrogenic cytokines, especially interleukin (IL)-1α, IL-1β, and tumor necrosis factor (TNF)-α, in response to a variety of conditions including infections, inflammation, trauma, or immunologic conditions.10 The production of these cytokines results in a cascade of effects, including the production of acute-phase proteins and stimulation of the immune response while also resulting in behavioral changes such as increased sleep, reduced appetite, and separation from others that may allow recovery and reduced spread of infectious agents.
Hyperthermic neonates often develop tachypnea to increase evaporative heat loss. Heat loss from the body surface, aided by peripheral vasodilation, is limited to the small areas in the axillary and inguinal areas, where less hair coverage is present. When lying in closed sternal recumbency, heat loss from these areas is minimized because of lack of conductive loss from circulating air. Affected individuals are lethargic. At rectal temperatures higher than 41.5°C (106.7°F), the body’s normal thermoregulation fails, peripheral vasoconstriction occurs, and cardiac output falls with reduction in blood pressure.10 Organ failure, coagulopathy, and myocardial necrosis may develop subsequently. Seizures may occur at these temperatures because of alterations in the function of temperature-sensitive ion channels and also the production of the pyrogenic cytokine IL-1β that has been shown to enhance neuronal excitability.14 Relatively small increases in brain temperature predispose the brain to hypoxic injury.8
If hyperthermia is identified in a neonate, it is likely to be significant, and therefore the clinician should attempt to identify the underlying cause and provide the appropriate treatment. It is particularly important to rule out or identify bacterial infection, since mortality rates may be high if this is not treated early and aggressively (see “Sepsis” below). Neonates that are suffering from heat stress as a result of environmental conditions should be cooled. This may be achieved by removing the cria from the heat source, making use of fans, clipping of fleece, and using ice packs. Ice packs may be particularly effective if placed in the inguinal area close to the femoral arteries and veins; care should be taken, however, to wrap the ice packs in towels and not to place them directly onto the skin, as this may cause cold burns. NSAIDs are also indicated for neonates suffering from heat stress or seizures caused by hyperthermia. The use of NSAIDs for reducing fever from other causes is controversial because of the beneficial effects that fever may have, including stimulation of the immune response, as well as the potential harmful GI and renal effects of NSAIDs. They should therefore be used with care.
Sepsis
Sepsis is defined as systemic inflammatory response syndrome (SIRS) with either documented or suspected infection by bacterial, viral, fungal or rickettsial agents.2 In crias, gram-negative and gram-positive bacterial sepsis appear to be the most common causes.2,3,12,15 E. coli, Pseudomonas, β-hemolytic Streptococcus, Enterococcus spp., Listeria monocytogenes, and Citrobacter are the most common isolates, with gram-negative organisms making up 54% of diagnoses in one study and 72% in another review.2,16 Most organisms are considered opportunists and infect individual crias, as opposed to causing outbreaks of disease. Neonatal sepsis is a common sequel to FPT and hence is common in crias after difficult births and in those with agalatic dams or with other risk factors contributing to FPT. It may also occur in crias shown previously to have acceptable passive transfer.
Sepsis is characterized by weakness, lethargy, pyrexia or, more commonly, hypothermia, tachycardia, tachypnea, and failure to nurse or gain weight.2,3 Evidence of dehydration, injected mucous membranes, or organ-specific signs, including diarrhea, colic, abdominal distention, hypopyon, seizures, blindness, ataxia, dyspnea, dysuria, lameness, or swollen joints, may exist. Signs may spread from one body system to another and may occur peracutely or gradually. When sepsis occurs before or soon after birth, it may be difficult to differentiate from dysmaturity, birthing complications, or disorders caused by birth defects, all of which may lead to severe obtundation or organ-specific signs. The greater the separation from the birth event, especially in crias that have shown days to months of normalcy, the more likely it is that local or systemic infection is the root of the problem. Generally, sepsis should be considered a possible cause or complication of almost any disease or disease sign in a neonate.
Initial physical examination findings are often relatively unremarkable, emphasizing the need for laboratory and possibly imaging evaluation of many sick neonates. Hematologic abnormalities often include leukocytosis or leukopenia, both often accompanied by a left shift and toxic changes in leukocytes. Neutropenia appears to be more common with gram-negative infections, whereas gram-positive infections are more commonly associated with neutrophilia, unless the infections are overwhelming.2 Blood work may also reveal findings suggestive of FPT, including hypoproteinemia and hypoglobulinemia. Specific tests of blood Ig concentrations provide more specific evidence. Measurement of total blood protein is not a particularly good predictor of FPT in septic crias but still provides useful information on whether a sick cria is hypoproteinemic or not.2 Additional blood abnormalities that may develop with sepsis include hypoglycemia or hyperglycemia, metabolic acidosis as a result of shock with or without diarrhea, azotemia as a result of dehydration, nephritis or kidney failure, hypoxemia or respiratory acidosis as a result of pulmonary infection or weakness-associated hypoventilation, electrolyte abnormalities, and increases in bilirubin and liver enzymes. Analysis of other body fluids, including peritoneal, pleural, joint, or cerebrospinal fluid (CSF), may also reveal local evidence of infection, if that part of the body is involved.
A broad-spectrum antimicrobial regimen is usually appropriate, with particular consideration for gram-negative coverage. Combination therapy of a β-lactam agent (crystalline penicillin: 22,000 units/kg. IV, q6h; or ceftiofur: 8 mg/kg, SQ, intramuscularly [IM], or IV, q12h) with an aminoglycoside such as gentamicin (5 mg/kg, IV, q24h for 5 days)17 or amikacin (15 to 18 mg/kg, q24h for 5 days) has seen widespread usage and resulted in many clinical cures. Other agents or combinations have not been as extensively used but may be supported by specific culture results. Antibiotics may have to be continued longer than suggested above, if sepsis persists. When using an aminoglycoside, monitoring of blood creatinine concentrations, preferably at least every 48 hours during treatment, may allow timely recognition of renal damage before it is irreversible.
Respiratory Dysfunction
Any evidence of insufficient respiratory function is usually considered the most urgent problem in a critical patient because of the potential for inadequate tissue oxygenation. Respiratory function may be affected by a variety of factors, including respiratory tract or cardiovascular anatomy; the presence of abnormal fluids, tissues, or infection affecting areas of air movement or gas exchange; mental or physical impairment of ventilation; abnormal blood flow patterns through the lung; and other factors. Some of these factors are more likely to be identified during the neonatal period than in older animals, particularly those relating to anatomic defects or infection. Assessment of the airway and respiratory function requires a thorough physical examination, imaging studies of the upper airway or chest, and ABG analysis.
Dyspnea, tachypnea, and open-mouth breathing are common in neonatal camelids with either an upper airway obstruction or fluid in their lungs but may be transiently present in healthy crias as well (Figure 42-4). They should resolve within an hour in healthy crias. If pathologic, these problems are usually the most urgent and demand immediate attention. Simple obstructions have been described earlier in this chapter, and anatomic airway defects are covered in Chapter 37. Trying to pass a feeding tube or rubber catheter may quickly yield information about airway patency and may remove mucus. Imaging studies are necessary for more thorough assessment. Respiratory obstructions should be removed or bypassed, if possible. This may require surgical intervention.
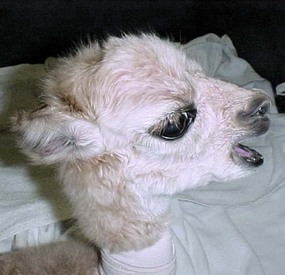
Figure 42-4 Open-mouth breathing. This may last a few hours in normal newborns, or may be a sign of congenital airway malformation or lower respiratory tract disease. Note also the curled ears characteristic of prematurirty or dysmaturity.
South American camelids are known to have some unique adaptations that allow them to thrive in low oxygen environments such as those found at high altitude. Their oxyhemoglobin dissociation curve is shifted to the left of those of humans and many other mammals, so camelid hemoglobin (Hb) has increased affinity for oxygen in the lungs, allowing high oxygen saturation of Hb (≥90%) even at high altitude and low inspired oxygen concentrations.18–20 However, the oxygen is released just as easily in the tissues, reflecting good oxygen extraction to meet the demands of tissue hypoxia. Fetal llamas have been shown to have even more efficient tissue oxygen extraction.21 Llamas and alpacas have been shown to exhibit a much reduced pulmonary vasoconstrictive response to hypoxia such that they are resistant to the development of pulmonary hypertension at altitude.22 Furthermore, the fetal llama, in contrast to lowland species, has been shown to respond to acute hypoxia with intense peripheral vasoconstriction and only a modest increase in cerebral blood flow with reduced oxygen consumption, indicating hypometabolism, even in the brain.23 Presumably, the large decrease in peripheral oxygen consumption preserves available supplies for the brain. The fetal llama heart also receives an increase in blood flow in response to hypoxia. These fetal responses appear to be preserved into the neonatal period.24 Therefore, the physiologic adaptations of these species may explain why neonates are better able to survive hypoxia in association with congenital heart defects compared with other domestic species.
Ventilation and oxygenation are best assessed via ABG analysis. If this is unavailable, or during the gap between presentation and confirmation of abnormalities, supplemental oxygen may be administered on the basis of suspicion of hypoxemia. If the patient is breathing poorly or has hypercapnea, mechanical or assisted ventilation may be required, if other treatments do not improve the respiratory effort. Generally, supplemental oxygen is recommended whenever partial pressure of oxygen (PaO2) falls below 60 mm Hg and ventilation is recommended whenever partial pressure of carbon dioxide (PaCO2) exceeds 60 mm Hg. Supplemental oxygen may be administered using oxygen tents or cages or more commonly via nasal catheter. It is usually sufficient to administer 2 to 4 liters per hour (L/hr) of 100% oxygen. This is effective or partially effective at combating hypoxemia caused by diffusion barriers and ventilation–perfusion mismatches but is not effective when blood is bypassing the lung because of a right-to-left shunt. Efficacy may be checked by comparing ABG values on and off the supplemental oxygen; a good response suggests a more favorable prognosis. The flow rate may be decreased and oxygen finally discontinued when clinical response warrants it.
Cyanosis is an additional sign of poor respiratory function. This bluish discoloration of the mucous membranes and the extremities is caused by accumulations of blood deoxyhemoglobin that exceed 2.5 grams per deciliter (g/dL). In addition to pulmonary and airway problems, cardiac defects causing blood to bypass the lungs are important contributors to cyanosis. These may include ventricular septal defect in combination with a right-sided obstructive lesion that results in right-to-left shunting, right-sided defects such as pulmonic stenosis with a patent foramen ovale, tetralogy of Fallot, transposition of the great vessels, or tricuspid atresia. These are covered in detail in Chapter 36.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree