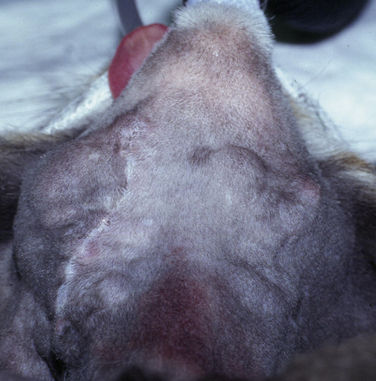
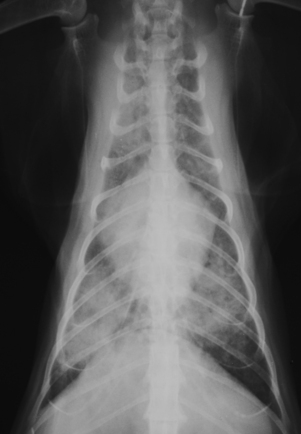
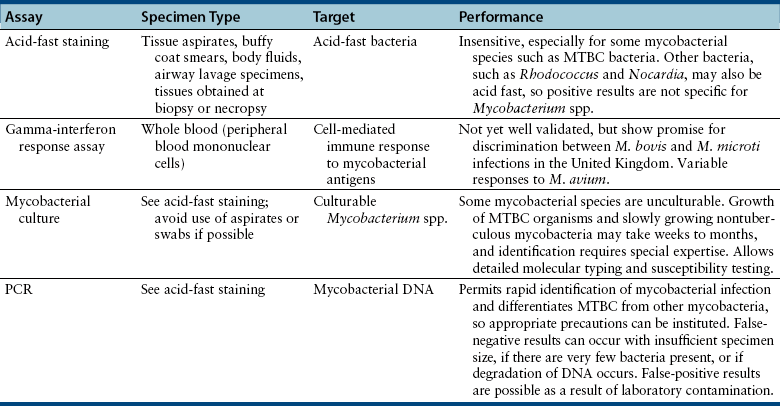
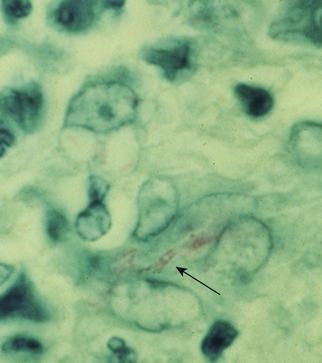
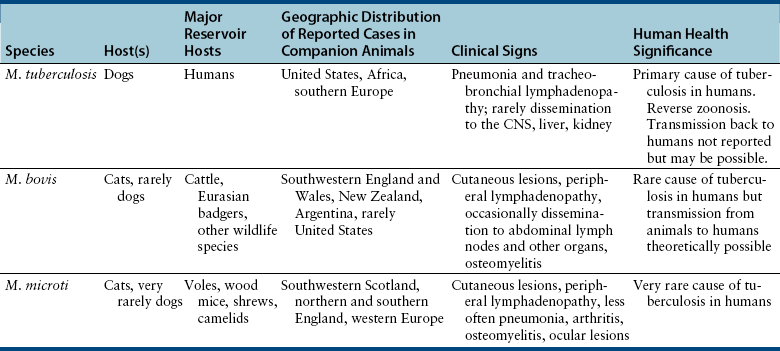
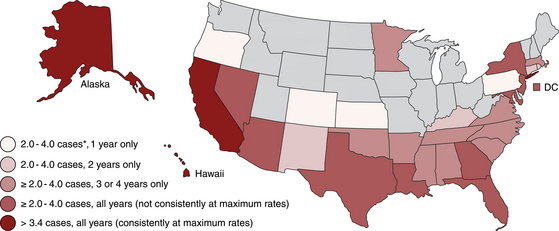
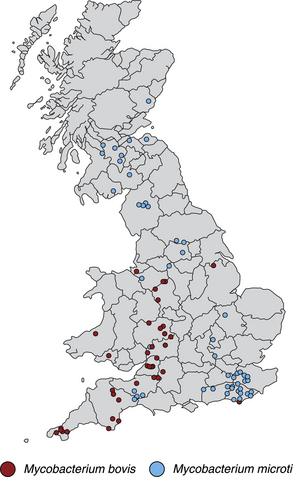
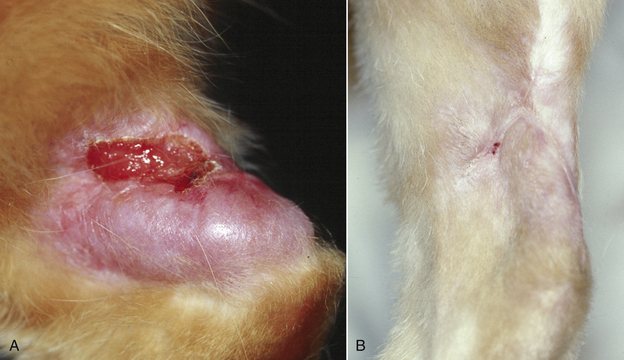
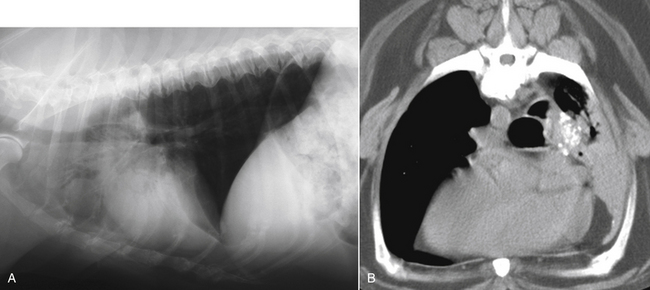
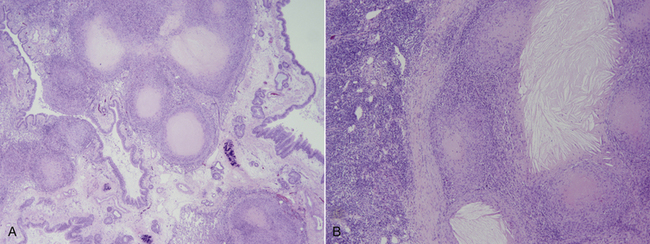
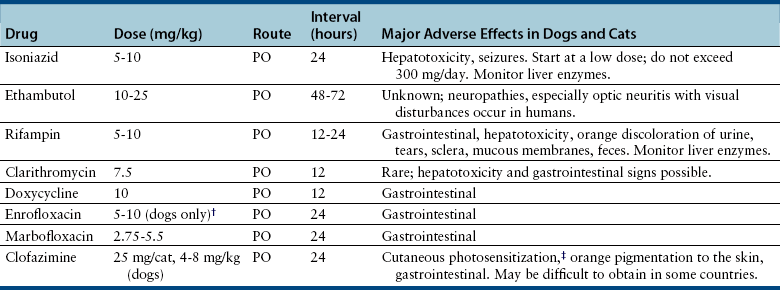
Chapter 44 Mycobacterium spp. are aerobic, nonmotile, non–spore-forming, gram-positive, acid-fast pleomorphic bacilli that cause chronic infections in humans and animals. Mycobacterium spp. have a high cell wall mycolic acid content, which causes them to retain a pink color when stained with acid-fast stains such as Ziehl-Neelsen or Kinyoun stains and examined under the microscope. As a result, the term “acid-fast bacilli” (AFB) is often associated with mycobacteria, although Nocardia is variably acid fast (see Chapter 43). Mycobacterium spp. belong to the family Mycobacteriaceae and are grouped into those that belong to the Mycobacterium tuberculosis complex (MTBC), the Mycobacterium avium complex (MAC), the lepromatous mycobacteria, and nontuberculous mycobacteria other than M. avium (Table 44-1). Disease caused by these groups of microorganisms differs in its epidemiology, pathogenesis, clinical presentation, zoonotic potential, and response to antimicrobial treatment. Accordingly, each group is considered separately in this chapter. TABLE 44-1 Mycobacterial Pathogens of Clinical Significance in Dogs and Cats CLG, Canine leproid granuloma. ∗The exact position of ‘M. visibile’ is not clear; it has also been grouped within the lepromatous mycobacteria. MTBC bacteria include the slowly growing, host-associated bacteria Mycobacterium tuberculosis, Mycobacterium bovis, Mycobacterium microti, Mycobacterium africanum, Mycobacterium pinnipedii, Mycobacterium caprae, and Mycobacterium canettii. These organisms cause tuberculosis in humans and animals. Only M. tuberculosis, M. bovis, and M. microti infect dogs and cats (Table 44-2). M. africanum and M. canettii are rare causes of human tuberculosis in Africa, M. pinnipedii infects seals, and M. caprae is primarily a ruminant pathogen. MTBC organisms share greater than 95% DNA-DNA homology, and so are difficult to differentiate from one another, even with many molecular methods. Unlike other mycobacterial species, MTBC bacteria do not survive well in the environment, although M. bovis can survive months in feces or carcasses. TABLE 44-2 Organisms of the Mycobacterium tuberculosis Complex of Clinical Significance in Dogs and Cats Humans are the only reservoir host for M. tuberculosis, which is responsible for more than 90% of tuberculosis in humans. Cats are resistant to infection with M. tuberculosis. Disease due to M. tuberculosis has been reported in dogs that reside in various parts of the United States, Africa, France, and Switzerland.2–9 Affected dogs from Europe have had travel histories; one was from western Africa and the other was a stray from southern Europe.2,3 Dogs are usually infected after prolonged aerosol exposure to contaminated human respiratory secretions, so the disease is a “reverse zoonosis” (or anthropozoonosis). Thus, disease occurs in dogs where tuberculosis occurs in humans. In the United States, M. tuberculosis infection is most prevalent in humans from the northeast/mid-Atlantic states, southern states, Alaska, California, and Nevada (Figure 44-1). Dogs have not transmitted M. tuberculosis infection to humans despite prolonged close contact in some situations,7 possibly because the pulmonary cavitary lesions that are required for transmission do not develop to the same extent in dogs as they do in humans. However, organisms have been detected in dogs’ sputum, so transmission remains possible. FIGURE 44-1 Geographic distribution of human cases of Mycobacterium tuberculosis infection in the United States, 2005-2011. Compiled from Morbidity and Mortality Weekly reports data on trends in human tuberculosis, years 2005, 2007, 2008, 2010, and 2011. ∗The intensity of the red color reflects the absolute number of cases/year, and whether case numbers were persistently high or varied among the 5 years evaluated. Thus the states that persistently have the highest rates of M. tuberculosis infection are Alaska, Hawaii, California, and New York (Long Island). Cats are most often infected with M. microti or M. bovis.10 Rodents (especially voles and shrews) are reservoir hosts for M. microti, and cattle and a variety of wildlife species are reservoir hosts for M. bovis. The overwhelming majority of feline infections with M. microti and M. bovis have been reported from the United Kingdom, where M. microti infection is endemic in voles, wood mice, and shrews, and M. bovis infection is endemic in Eurasian badgers and cattle. Infections with M. microti and M. bovis comprise a third of feline infections with culturable mycobacteria in the United Kingdom.10 Cats infected with M. bovis tend to be young adult cats (median age 3 years), whereas the median age of cats infected with M. microti is 8 years.10 All affected cats have been more than 1 year of age, which likely reflects the long incubation period associated with these infections. Retroviral infection does not appear to predispose to infection. M. bovis infections mainly occur in cats that reside in Wales and southwestern England and that have access to the outdoors (Figure 44-2).10 Feline M. bovis infections also occur in New Zealand, where brushtail possums act as reservoir hosts11; Argentina, where stray cats are often fed raw cattle lung12; and rarely the United States.13 In the United Kingdom, spillover of infection from cattle, badgers, and possibly small rodents to cats is suspected. Aggressive interactions between cats and infected badgers may also be a route of transmission, especially as most cutaneous lesions occur at sites where fight and bite wounds often occur.10,14 Ingestion of unpasteurized milk by cats is no longer a common route of transmission. A study from the United States showed no evidence of transmission of M. bovis to cats or dogs that lived on infected cattle farms.15 Major wildlife reservoirs in North America, which include white-tailed deer, bison, and elk, are less likely than small mammals to interact with cats, which may explain why fewer M. bovis infections are reported in cats from North America when compared with the United Kingdom. Dogs are uncommonly infected with M. bovis.16–18 Wounds inflicted by badgers or a squirrel have preceded disease in affected dogs.16 In recent years, disease in dogs has been restricted to southwestern England or Ireland. FIGURE 44-2 Geographic distribution of Mycobacterium bovis and Mycobacterium microti isolates from cats in the United Kingdom. (Redrawn from Gunn-Moore DA, McFarland SE, Brewer JI, et al. Mycobacterial disease in cats in Great Britain: I. Culture results, geographical distribution and clinical presentation of 339 cases. J Feline Med Surg 2011;13:934-944.) M. microti infections mainly occur in cats with outdoor access from the United Kingdom, with major clusters in south-western Scotland/northern England and southern England (see Figure 44-2).10,19 Spillover of disease from voles to cats is suspected.19 Vole and llama genotypes of M. microti have been identified. Cats in the United Kingdom and one in Switzerland have been infected with the vole type.20 A dog from France was infected with the llama type.21 Clinical signs in dogs with pulmonary tuberculosis due to M. tuberculosis are slowly progressive. They consist of chronic, productive, or nonproductive cough, as well as inappetence, weight loss, and lethargy.4,7 Rarely, dissemination to nonpulmonary sites such as the central nervous system (CNS), liver, or kidney leads to neurologic signs, weight loss, vomiting, or diarrhea.2,3,9 Involvement of the lungs, liver, kidneys, and/or lymph nodes can occur in dogs infected with M. bovis.16,18,22 Isolated lung and tracheobronchial node involvement similar to that often reported for M. tuberculosis was described in one dog that was infected with M. bovis.18 Most (>75%) cats infected with M. bovis and M. microti have had cutaneous nodular lesions with or without mandibular lymphadenopathy. Multifocal peripheral lymphadenopathy may be present.23 Mesenteric lymphadenopathy, pneumonia, ocular lesions, and arthritis occur in some cats infected with M. microti.20,23–25 Dissemination may result in weight loss, fever, and inappetence. Lung involvement may be associated with tachypnea, dyspnea, or cough.18 The most common physical examination findings in cats (and to a lesser extent, dogs) that are infected with M. bovis or M. microti are single or multiple firm cutaneous nodules, and enlargement of one or more peripheral lymph nodes (Figures 44-3 and 44-4). Cutaneous nodules may be mobile or fixed to underlying tissues (muscle and bone) and can ulcerate or drain purulent fluid. Skin lesions are most prevalent on the head but can also occur on the limbs and the trunk.20,23 Enlarged mesenteric lymph nodes, spleen, and/or liver may be detected on abdominal palpation. Involvement of internal lymph nodes is more common with M. bovis infections (21% of cats) than with M. microti infections (9% of cats).10 Lameness may be present in cats with skeletal involvement. FIGURE 44-3 Ulcerative skin lesion due to Mycobacterium microti infection in a 6-year-old male neutered domestic shorthair. A, Before treatment. B, After 2 months of treatment with a combination of marbofloxacin, azithromycin, and rifampin. Diagnosis of MTBC infection relies on suggestive history, clinical signs, and radiographic abnormalities, combined with the results of histopathology, molecular tests, and culture. When mycobacterial infection is suspected, clinical specimens should be divided into portions—one for histopathology (in formalin), one for mycobacterial culture, one for routine cultures for other bacterial species, and one for mycobacterial PCR (fresh or frozen). The laboratory should be informed that mycobacterial infection is a possibility. Complete Blood Count, Serum Biochemical Tests, and Urinalysis Laboratory abnormalities in dogs and cats infected with MTBC organisms are nonspecific. Mild anemia of inflammatory disease and neutrophilic leukocytosis, sometimes with a left shift, and monocytosis are often present. The serum chemistry profile may show hypoalbuminemia and hyperglobulinemia. Hypercalcemia of granulomatous disease can also occur in dogs or cats.10,17 Increased serum liver enzyme activities have been reported in dogs with hepatic involvement.3 Findings on plain thoracic radiography in dogs infected with M. tuberculosis or M. bovis include tracheobronchial or mediastinal lymphadenopathy,3,4,7 interstitial to alveolar infiltrates,22 pleural effusion,3 and lobar consolidation (Figure 44-5A).7,22 Similar abnormalities can occur in cats infected with M. microti and M. bovis (Figure 44-6). Bronchial, alveolar, nodular interstitial, and unstructured interstitial patterns predominate in cats, and perihilar lymphadenopathy can be present.20,26 Calcification of pulmonary lesions or lymph nodes may be evident in dogs or cats. Hepatomegaly, splenomegaly, or lymphadenopathy, with or without ascites, may be present on abdominal radiography, or abdominal radiographs may be unremarkable.26 Radiographic evidence of osteomyelitis (especially osteolysis) can occur in cats infected with M. microti or M. bovis, often in association with overlying cutaneous lesions.26 FIGURE 44-5 Lateral thoracic radiograph (A) and 7-mm collimated computed tomography (CT) image immediately cranial to the carina (B) from a 9-year-old female spayed golden retriever with pulmonary tuberculosis. A, Consolidation of the right cranial lung lobe is present together with interstitial to alveolar infiltrates in the right middle lung lobe. B, On the CT scan, the right cranial lung lobe is consolidated and markedly volume reduced. Air bronchograms are present centrally that extend dorsally toward a focal region of aerated lung. The granular mineral opacity adjacent to the right margin of the trachea represents mineralized lymphadenopathy of the right tracheobronchial lymph nodes. (From Sykes JE, Cannon AB, Norris AJ, et al. Mycobacterium tuberculosis complex infection in a dog. J Vet Intern Med 2007;21:1108-1112.) Thoracic CT findings have been reported for a dog infected with M. tuberculosis.7 Lung lobe consolidation, multifocal stellate opacities, bronchial stenosis, bronchiectasis, tracheobronchial wall thickening, and partially mineralized masses consistent with enlarged mediastinal and tracheobronchial lymph nodes were present (Figure 44-5, B). In this dog, the mineralization was not visible on plain thoracic radiographs. CT in human patients with tuberculosis is considered more sensitive than plain radiography for detection of pulmonary lesions and enlarged lymph nodes and provides better visualization of calcifications. Abdominal sonography in animals with MTBC infections may be unremarkable or reveal hepatomegaly, splenomegaly, abdominal lymphadenopathy, and/or ascites. A dog with disseminated M. tuberculosis infection had multifocal hypoechoic masses within the kidneys and liver and mineralization of the hepatic and renal parenchyma.4 Cytologic examination of aspirates, effusion, or lavage specimens from the respiratory tract of dogs with pulmonary tuberculosis typically reveals a mixed population of often heavily vacuolated histiocytes and smaller numbers of degenerate or nondegenerate neutrophils and small lymphocytes.3,7,22 Intracytoplasmic, nonstaining bacilli were seen in macrophages in a bronchoalveolar lavage specimen from a dog infected with M. bovis.22 Moderate anisocytosis or anisokaryosis of exfoliated respiratory epithelial cells can be present, which might cause suspicion for pulmonary neoplasia.7 The presence of cholesterol crystals, caseous debris, and concentrically laminated crystalline structures known as calcospherite bodies can be a clue for the presence of MTBC infection.7,22 Assays available for diagnosis of mycobacterial infections are listed in Table 44-3. Intradermal inoculation of tuberculin (a purified protein derivative from M. tuberculosis), or the Mantoux test, is well validated and widely used in human medicine for diagnosis of, and screening for, infection with MTBC organisms. Infected individuals develop a cutaneous DTH reaction at the site of inoculation 48 to 72 hours later. False-negative test results can occur as a result of underlying immunosuppression. False-positive results occur as a result of nontuberculous mycobacterial infection. Cats do not respond reliably to tuberculin skin testing. In dogs, tuberculin testing has been performed on the medial surface of the pinna, but false-positive results have been documented with a variety of other bacterial infections. Tests for anti-mycobacterial antibodies are also not specific.27 Cell-based assays (ELISA and ELISPOT), which measure IFN-γ production by patient peripheral blood mononuclear cells in response to specific mycobacterial antigens, have been used for diagnosis of tuberculosis in humans and cattle. These assays have been adapted for use in cats and have the potential to rapidly distinguish infection with M. bovis from that with M. microti when responses to different mycobacterial antigens are compared.28 Routine aerobic, anaerobic, fungal, and mycoplasma cultures are negative when performed on specimens collected from animals with MTBC infections. Mycobacterial culture is required and is the gold standard for diagnosis of MTBC infection. The use of laboratories with expertise in isolation of MTBC is recommended. Respiratory specimens such as respiratory lavages are usually treated with the mucolytic N-acetylcysteine and a sodium hydroxide solution to destroy contaminating bacteria. Specimens from normally sterile sites, such as pleural fluid, are not decontaminated so that viable mycobacteria are preserved as much as possible. Specimens are then inoculated onto special liquid or solid media for isolation of mycobacteria, such as 7H11 agar or Lowenstein-Jensen media (an egg-based medium). Modifications to the media composition may be required on the basis of the mycobacterial species suspected, so good communication between the clinician and the microbiology laboratory can optimize the chance of successful culture. Growth is evident 1 to 3 weeks after inoculation of liquid media, and 3 to 8 weeks after inoculation of solid media. However, inoculation of solid media is still required, because some strains grow only on solid media, and growth on solid media allows assessment of colony morphology. Once growth is evident, nucleic acid–based assays, mycolic acid analysis with high-performance liquid chromatography, or mass spectrometry (MALDI-TOF)29 can be used to determine whether the organism belongs to the MTBC. Antimicrobial drug susceptibility testing can also be performed. The most frequent gross pathologic findings in dogs infected with M. tuberculosis are pulmonary congestion, lobar consolidation, or granulomatous mass formation and sometimes massive enlargement of tracheobronchial and mediastinal nodes. Lymph nodes may contain multifocal gritty, yellow, caseous material.4,7,22 Pleural effusion may also be present.3 In dogs with disseminated disease, pale nodular lesions may be found in abdominal organs such the liver, peritoneal surfaces, and kidneys.2,4,22 In one unusual case, a dog from Africa had nodular lesions that were confined to the liver and brain.2 Dogs infected with M. bovis have abnormalities similar to those infected with M. tuberculosis.16–18 In addition to cutaneous lesions and peripheral lymphadenomegaly, cats with disseminated M. bovis or M. microti infections may have abdominal lymphadenomegaly and multifocal tumor-like, grayish-white masses within parenchymal organs that can have hemorrhagic margins and/or a soft purulent center. Pulmonary lesions are often grayish red and may be accompanied by serosanguineous pleural effusion. Histopathologic findings are similar for all MTBC diseases. Characteristic tubercles or granulomas are present in tissues, which vary in size and may coalesce (Figure 44-7). Tubercles consist of abundant epithelioid macrophages and lesser numbers of neutrophils. Multinucleated giant cells are rarely present in dogs or cats.4,30 The inflammatory foci are surrounded by a layer of macrophages, neutrophils, lymphocytes, and plasma cells and an outer layer (or background) of granulation tissue. The center of the tubercle may be necrotic. Central mineralization and lipid accumulation occurs in some dogs infected with M. tuberculosis,7,22 but mineralization is rare in cats infected with M. microti or M. bovis. Acid-fast stains reveal low numbers of AFB in tubercle centers in most cases, but a careful search may be required (Figure 44-8).4,7 FIGURE 44-7 Multifocal granuloma formation in the lung (A) and thoracic lymph node (B) of a 9-year-old female spayed golden retriever with pulmonary tuberculosis. The granuloma within the lymph node contains a lake of lipid and necrotic cellular debris, surrounded by an internal layer of epithelioid macrophages, a thick layer of fibrosis, and an external layer of lymphocytes, plasma cells, and histiocytes. Fite and Ziehl-Neelsen acid-fast stains revealed scant numbers of acid-fast positive bacteria in areas of central necrosis (not shown). Dogs and cats with suspected MTBC infection should be placed in isolation, and aerosol precautions are indicated (see Chapter 11). Treatment of dogs and cats with MTBC infection is controversial because of concerns that relate to zoonotic potential. It should only be performed after owners have been fully educated about the potential risks of disease transmission. Most affected dogs have been euthanized without specific treatment, but occasionally euthanasia of animals with non-MTBC mycobacterial infection has occurred before a diagnosis of MTBC was confirmed. Combination drug therapy with the antimycobacterial drugs ethambutol, isoniazid, rifampin, and pyrazinamide is used to treat human patients. Ethambutol and isoniazid interfere with mycobacterial cell wall synthesis; the mechanism of action of pyrazinamide is not known, and M. bovis is naturally resistant to it. Treatment of a dog with M. tuberculosis infection was attempted with rifampin, isoniazid, and clarithromycin, but the dog was euthanized after seizures developed.7 The seizures were suspected to be an adverse effect of isoniazid administration. Successful treatment of cats with cutaneous and/or pulmonary MTBC infections has been achieved with combinations of fluoroquinolones, clarithromycin or azithromycin, doxycycline, and a rifamycin; most successfully treated cases have received a fluoroquinolone, clarithromycin or azithromycin, and a rifamycin (Table 44-4). Surgical excision of small skin lesions can be curative if dissemination is not present, but lesions may dehisce and progress if resection is not complete, and it may not always be possible to determine whether dissemination has occurred. The use of three drugs has been recommended for the first 2 months of treatment (e.g., rifampin, clarithromycin, and a fluoroquinolone), followed by a continuation period with two drugs (usually the macrolide and the fluoroquinolone, because rifamycins are more often associated with adverse effects). Esophagostomy tube placement may be necessary to facilitate treatment and reduce the chance of exposure of the owner to the infection through bite or scratch wounds. TABLE 44-4 Suggested Drug Doses for Treatment of Mycobacterial Infections∗ ∗See text for recommended drug combinations for each mycobacterial species and additional drugs that may be useful. †The use of other fluoroquinolones such as marbofloxacin or pradofloxacin is preferred in cats because of the risk of retinal toxicity (see Chapter 8). ‡Bennett S. Photosensitisation induced by clofazimine in a cat. Aust Vet J 2007;85:375-380.
Mycobacterial Infections
Group
Mycobacterial Species
Description
M. tuberculosis complex
M. tuberculosis, M. bovis, M. microti
Host-associated, slow-growing species. Cause cutaneous and disseminated granulomatous disease in dogs (all three species) and cats (M. bovis and M. microti)
M. avium-intracellulare complex
M. avium subsp. avium, M. avium subsp. hominissuis
Opportunistic, environmental, slow-growing species. Cause cutaneous and disseminated granulomatous disease in dogs (subsp. hominissuis) and cats (subsp. avium). Some may cause leprosy syndromes in cats.
Lepromatous mycobacteria
M. lepraemurium, the CLG organism, and other unnamed species, some with genetic resemblance to known nontuberculous mycobacteria
Highly fastidious or unculturable mycobacteria. Cause nodular granulomatous to pyogranulomatous cutaneous disease in cats (all species) or dogs (CLG organism).
Rapidly growing nontuberculous mycobacteria
M. fortuitum, M. smegmatis, M. abscessus, M. chelonae, M. thermoresistibile, M. goodii, M. flavescens, M. alvei
Opportunistic, environmental, rapidly growing species. Cause pyogranulomatous panniculitis, pneumonia, or disseminated infections in cats or dogs
Slowly growing nontuberculous mycobacteria other than M. avium
M. kansasii, M. ulcerans, M. genavense, M. malmoense, M. celatum, M. terrae, M. simiae, ‘M. visibile’∗
Opportunistic, environmental, slowly growing species. Cause pyogranulomatous cutaneous or disseminated disease in dogs or cats.
Mycobacterium tuberculosis Complex
Clinical Features
Physical Examination Findings
Diagnosis
Laboratory Abnormalities
Diagnostic Imaging
Computed Tomography
Sonographic Findings
Cytologic Examination
Microbiologic Tests
Immunologic Assays
Bacterial Isolation and Identification
Pathologic Findings
Histopathologic Findings
Treatment and Prognosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree