Fig. 1.
Macroscopical view of the brain, in a case of ENU-induced brain tumor. The left hemisphere shows an edematous aspect due to the presence of the intracerebral tumor (arrow).
Unequivocal ependymomas were not seen in series of mice exposed transplacentally to ENU, but according to accepted classifications, approximately 20% of the ENU-induced brain tumors could be diagnosed as ependymomas, anaplastic ependymomas, or mixed glial tumors with ependymoma areas (24). In many classifications, ependymomatous tumors were termed as “anaplastic glioependymomas” due to the presence of ependymoma-like cells, with other glial-like cells, pleomorphic cells, and generally with rounded cells very similar to those of human oligodendrogliomas.
In 1973, Jones et al. (22) distinguished five groups of ENU-induced tumors which were, in order of frequency: periventricular subependymal plate gliomas, astrocytic and oligodendrocytic gliomas, neuronal tumors of the spinal and intracranial ganglia and nerves, neuronal tumors of the central nervous system, and meningeal tumors. Periventricular subependymal plate gliomas were further divided into ependymomas and mixed ependymo-oligodendroastrocytomas, these latter tumors being equivalent to the anaplastic glioependymomas of Koestner et al. (26). In these tumors, the neoplastic ependymomatous cells are arranged in groups, cords, and rosettes or packed in solid masses. The majority of cells are medium or large, with oval or irregular nuclei showing prominent nucleoli. The cytoplasm varied from being abundant to forming a narrow ring around the nucleus. Mitosis and vascular hyperplasia is frequent, with hemorrhagic and necrotic areas, especially in large tumors. Ultrastructurally, two cell types predominate, a smaller, undifferentiated, and another cell type, showing greater size and nuclear pleomorphism (11, 24, 25). However, in these cells, generally there are no characteristics of ependymoma cells, such as the presence of cilia or specialized cell junctions.
In any case, studies on the early stages of the tumor development suggest that most, if not all, ENU-induced brain gliomas originate from the subependymal plate (11), a layer of primitive cells which exists throughout postnatal life in mammals as a remnants of the periventricular matrix, and represents an area of neurogenesis in adults. After the interaction of the carcinogen with cells in this region, the transformed cells can migrate into the white matter, immediately beneath the cortex, or remain in situ. As a result of neoplastic transformation, neural stem cells of the subependymal plate are affected, and the predominance of cells showing differentiation toward a glial phenotype or the other determines the different morphology of each tumor, explaining the frequent heterogeneity of cell population in the ENU-induced gliomas.
In 1992, we studied the immunohistochemical expression of synaptophysin in seven experimental brain tumors previously classified as anaplastic ependymoma or mixed oligodendroglioma-ependymoma on the basis to their histologic pattern (6). The seven brain tumors were induced by transplacentary administration of ENU (50 mg/kg) to adult Wistar rats, at day 17 of gestation, and all of them were clinically manifested in the offspring by the appearance of neurologic signs after a mean latency of 10 months. Our results showed strong synaptophysin-positivity in most of the tumor cells (Fig. 2), and electron microscopy revealed rosettes of neuroblastic type and tumor cells processes with microtubules, electron-dense bodies, and dense core vesicles, without features that characterize ependymomatous tumors. These findings support the opinion that ENU-induced tumors are undifferentiated neuroepithelial tumors, regardless of their location or morphology (7–9).
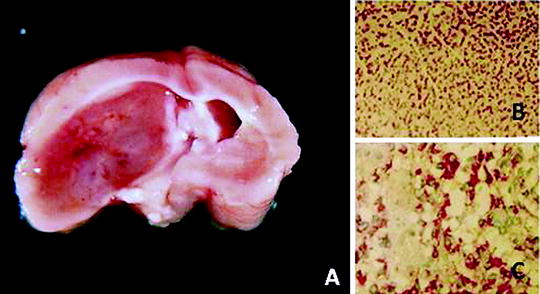

Fig. 2.
(a) Macroscopic aspect of an ENU-induced brain tumor in the Wistar rat. The tumor was classified as a malignant ependymoma. (b) Histological aspect of the tumor (×100) (H.E.). (c) Strong synaptophysin expression in the tumor cells (×200).
5 The Ependymoblastoma Murine Model
Ependymoblastoma is considered in the actual WHO classification of human brain tumors as a rare, malignant, and embryonary tumor, manifestating in neonates and young children, and histologically characterized by distinctive multilayered rosettes (29). Nevertheless, in the past, the term “ependymoblastoma” has been used in human pathology for the description of malignant ependymomas or undifferentiated malignant gliomas containing areas with ependymomatous aspect. This fact explains the description as ependymoblastoma of experimental brain tumors consisting of malignant undifferentiated cells and forming the pseudorosettes that usually can be observed in ependymomatous tumors.
In 1939, Seligman and Shear induced brain tumors in mice, through the application of methyl cholantrene (11), and 2 years later, Zimmerman and Arnold (30) were able to transplant these tumors intracerebrally in other mices, obtaining growth in 90–100% of cases. From these original tumors, Ausman et al. (31) obtained several cell lines, including a line named as “murine ependymoblastoma A,” with histological structure that suggested a malignant and embryonary ependymomatous tumor. This line has been used in numerous studies of experimental neuro-oncology, due to its easy transplantation on the brain of recipient mices, but it lack many of the characteristics noted in the human ependymoblastomas or malignant ependymomas, including ultrastructural ependymal features and cell kinetics (32). Furthermore, the murine ependymoblastoma contains presumed endogenous retrovirus, murine mammary tumor particles, and murine C-type viral structural antigens (33), which has led some authors to suggest that it can actually be a virus-induced tumor (34).
6 Xenografts of Ependymomatous Tumors
The possibility to obtain xenografts of human brain tumors has been considered an acceptable model for studies in experimental neuro-oncology. However, it requires the implantation of the tumor on the nude mouse, a model characterized by the absence of immunological defense, which allows the development of xenografts, but interferes with many aspects of the neurobiology of brain tumors, in which, the immune response determines the outcome and prognosis (35).
Some authors have studied the growth and survival of human ependymomas xenografted to nude mouse. As an example, in 1996, McLendon et al. (36) reported nude mouse xenografts of human ependymomas obtained from two nonrelated childhood. Xenografts were performed in both subcutaneous and intracranial locations, with a median postimplantation survival about 3 months. Both xenografts grow as well-formed masses, with no evidence of infiltration into either brain or subcutaneous tissues. While perivascular pseudopalisading was found in both xenografts, true ependymal rosette formation was absent. Ultrastructurally, neither xenografts exhibited cilia, but both produced abundant intermediate filaments. Neoplastic cells showed positivity to glial fibrillary acidic protein (GFAP), vimentin, and nestin. Thus, it is obvious that xenografts of human ependymomas can be performed on the nude mouse model, which offers the possibility of several studies about the progression and therapy of these tumors.
In this same way of research, recently, Yu et al. (37) injected a fresh ependymoma specimen from a 9-year-old patient into the brain of RAG2/severe complex immune deficiency (SCID) mice. All five mice receiving the graft developed tumor growth, which were serially subtransplanted in vivo in mouse brains for four generations and can be cryopreserved for long term. Xenografts shared identical morphological features with the original tumors, and they contained a small population of CD133+ stem cells that can form neurospheras and display multipotent capabilities in vitro. The authors obtained a new and permanent cell line (BXD-1425EPN) that can facilitate further biological studies on ependymomas.
7 Conclusions
When the possibility to obtain an experimental model of ependymoma is considered, it is obvious that none of the animal models presently used fits the criteria for an ideal model. Genetically engineered animal models have been used in recent years to the study of brain tumors, but none of them can be considered useful as an experimental model of ependymoma. Viral neurocarcinogenesis obtains occasionally tumors with ependymoma aspect, but ultrastructural studies disclosed important questions about whether they really can be classified as ependymomas or choroidal plexus tumors. Most of the experimental cerebral neoplasms identified as “ependymoma” or “ependymoma-like” tumors are, in fact, malignant neuroectodermal tumors, and their intraventricular location or their histological features are not arguments supporting that they are tumors arising from ependymal cells. The named “murine ependymoblastoma” is a tumor of uncertain classification, whose origin is also controversial, and data exist for the possibility that it represents a virus-induced tumor. Finally, the possibility of using xenografts of human ependymomatous tumors on the model of nude mouse or other animal models presenting immune deficiency presents the difficulty of working on an experimental model in which the absence of host immune response may mask many issues that currently are considered important in the biology of brain tumors.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


