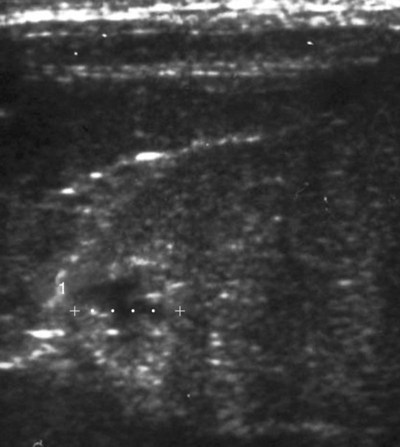
Melioidosis is an infection caused by the saprophytic beta-proteobacterium Burkholderia pseudomallei (formerly Pseudomonas pseudomallei), an aerobic, gram-negative, motile bacillus with a single polar flagellum. Its habitat is generally restricted to the environment at latitudes within 20 degrees north or south of the equator; however, its exact ecologic niche is yet to be identified. As such, it is endemic to Southeast Asia, northern Australia, and the South Pacific.16,68 Sporadic autochthonous cases are reported outside of the area of high endemicity, including the Caribbean, Central and South America, West and East Africa, the Indian subcontinent, and the Middle East.16 Multilocus sequence typing (MLST) of isolates, using the sequences of internal fragments of seven housekeeping genes, has provided valuable insights into the epidemiology and evolution of the organism.17,22,32,46 There are currently over 600 sequence types described for B. pseudomallei, and extensive molecular diversity of the organism is seen especially in the Northern Territory of Australia, where more than 250 separate sequence types have been identified.18 B. pseudomallei can be isolated most reliably from moist clay soils and pooled surface water from endemic areas. Human infection numbers increase after heavy monsoonal rains and winds, presumably because of inhalation of organism-laden aerosols.7,16,16 Infection is thought to occur via cutaneous inoculation through wounds contaminated with soil, or from bites of arthropod vectors; inhalation of dust, aerosols, or muddy water (such that occurs with near drowning); or ingestion of soil, contaminated infected carcasses, contaminated drinking water, or milk (following infection of the mammary gland).45 Because of the risks of inhalation or ingestion, the bacterium has been labeled as a biological weapon and has been classified as a Category B bioterrorism agent by the United States Centers for Disease Control and Prevention (CDC),10 although its true potential in this scenario is unclear. B. pseudomallei is a facultative intracellular organism, causing a variable spectrum of clinical syndromes in humans and animals including (1) mild or inapparent infections, (2) local skin ulcers, (3) chronic abscess formation and granuloma formation in subcutaneous tissues or internal organs, (4) chronic pneumonia, or 5) acute, fulminant septicemia with resultant pneumonia and/or encephalomyelitis. There has been no documented correlation between specific MLST results of given strains of the organism and the respective clinical presentation or severity of infection.17 B. pseudomallei generally has a low propensity to cause disease in clinically healthy hosts. Severe disease is mostly seen in individuals with compromised immunity, particularly those with impaired neutrophil function, such as with diabetes mellitus and chronic renal failure.47 The infection may remain subclinical for long periods, with the sudden appearance of disease after many years of dormancy, similar to that seen with tuberculosis. The longest recorded incubation period was in a U.S. veteran of the Pacific theater of World War II, who was diagnosed 62 years after presumed exposure.50 Innate immunity appears to be primarily important for protection against infection in the host. Major impairment of adaptive cell-mediated immunity, such as that seen in advanced human immunodeficiency virus infection, does not appear to be a risk factor for disease in humans.16 Likewise, a high antibody titer does not protect against either primary infection or relapse.16,41,41 Although reports of canine and feline melioidosis are infrequent in the literature, anecdotal evidence suggests that infection is more common.51 Seven military dogs stationed in Vietnam developed fever, myalgia, and abscess formation of multiple organs, including the dermis, lung, liver, kidneys, spleen, epididymis, and testis,63 and an Australian dog developed disseminated cutaneous melioidosis after ingesting offal from a goat that had died of the disease.44 Of 6 cats reported with melioidosis,20,51,53,67 3 were presumed to have acquired the infection in Malaysia, with the remaining 3 cases having domiciled in Northern Australia. Multifocal abscesses, lymphadenomegaly, hepatomegaly, and splenomegaly were most common in the affected cats (Fig. 44-1). The authors and their colleagues have documented infection in 2 cats with unilateral panophthalmitis.53 The preferred diagnostic method is isolation of the organism from bodily fluids or tissues. Generally, B. pseudomallei is grown on routine bacteriologic media, although selective media such as modified Ashdown’s agar may be needed to isolate the organism from sites where contaminating microorganisms are present. Thorough examination of Gram-stained smears of pus from lesions can sometimes identify small numbers of bipolar-staining gram-negative rods (often described as having a “safety-pin” appearance); this may increase suspicion of the infection in endemic areas (Fig. 44-2). Although culture of the organism is considered the gold standard for diagnosis, results are typically not available for 3 to 4 days. This delay may contribute significantly to the high mortality rate in fulminant cases. Alternative diagnostic modalities have been developed to augment culture methods in an attempt to improve patient outcomes. Immunologic assays are available for the detection of Burkholderia antigen in samples or culture supernatant and for antibodies in serum. However, none of these methods have been specifically adapted for use in dogs or cats. In humans, antibody detection may reflect past exposure rather than active disease, although a single titer at or above 640 is highly suggestive of current infection.43 Polymerase chain reaction and loop-mediated isothermal amplification can detect B. pseudomallei DNA in patient samples; however, both techniques have been found to have poor specificity and/or sensitivity, and neither is currently available commercially.11,12,12 A rapid oligonucleotide microarray assay has been used for accurate rapid identification of B. pseudomallei and distinguishing it from Burkholderia mallei.60a Pathologic findings are abscesses in affected organs and draining lymph nodes (Fig. 44-3). Immunohistochemical methods can detect the organism in affected tissues.69 In the veterinary setting, perhaps the best diagnostic aid is a high clinical suspicion, especially in areas where B. pseudomallei may be the most common cause of community-acquired sepsis in humans. Treatment of melioidosis may be prolonged and is often unsuccessful (mortality rates in human patients with fulminant melioidosis range from 20% to50%),23,28a,68 and relapses may occur. There are few reports of successful treatment in animals.45,53 Surgical drainage of large abscesses, or enucleation in the case of panophthalmitis, may be required in conjunction with antimicrobial therapy. Antibacterial treatment is recommended for small or disseminated lesions. The organism tends to be susceptible in vitro to trimethoprim-sulfonamides, amoxicillin-clavulanate, ticarcillin-clavulanate, some third-generation cephalosporins and quinolones, tetracyclines, chloramphenicol, and carbapenems.16,66 B. pseudomallei is generally resistant to penicillin G, aminopenicillins, first- and second-generation cephalosporins, most aminoglycosides (including gentamicin), most macrolides (including azithromycin), second-generation quinolones, and rifampin.14,16,39a Current recommendations for treatment of acute melioidosis in human patients are high doses of parenteral ceftazidime plus trimethoprim-sulfonamide given for 2 to 4 weeks or meropenem for at least 2 weeks, followed by a maintenance drug regimen of orally administered trimethoprim-sulfonamide, and doxycycline, with or without chloramphenicol, for 12 to 20 weeks.14a,36 During the acute stages, intravenous amoxicillin-clavulanate or ticarcillin-clavulanate might be combined with an initial concurrent course of a parenteral carbapenem (for example, imipenem or meropenem). Amoxicillin-clavulanate has also been shown to be adequate for maintenance treatment.56 Despite these antibacterial therapy recommendations, rigorous clinical studies addressing questions regarding optimal duration of intravenous therapy and ideal regimens for eradication of the organism are lacking. Adjunctive treatment with granulocyte colony-stimulating factor has helped reduce mortality in humans with septic shock due to melioidiosis15 and might be effective in veterinary patients. For appropriate therapeutic dosages, see the Drug Formulary in the Appendix. Following a response to treatment, affected animals should be closely monitored for signs of relapse. Nosocomial transmission of infection has been suspected.45 The organism has survived in a parenteral anesthetic solution and possibly an antiseptic cleaning agent containing cetrimide 3% and chlorhexidine 0.3%.45 Zoonotic transmission is thought to be extremely rare, although anecdotal reports of animal-to-human transfer have been documented.37,45 Generally, humans in endemic areas become infected from the environment, as do animals.
Miscellaneous Bacterial Infections
Melioidosis
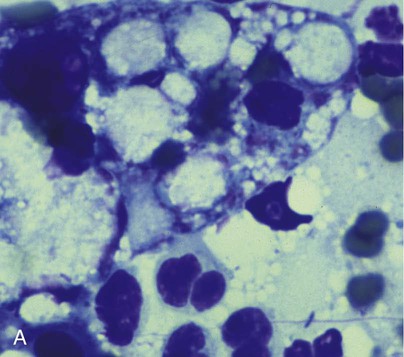
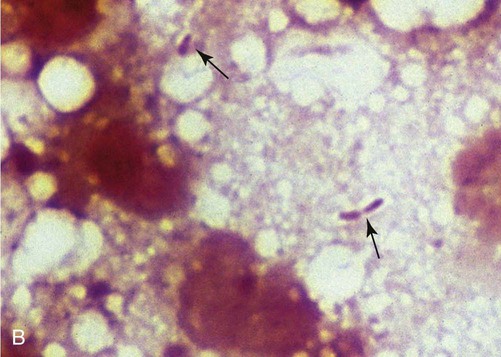
B, Squash preparation of liver lesion from cat with disseminated melioidosis obtained at necropsy. Note the extracellular gram-negative bacilli (arrows) (Burke’s modification of the Gram stain; original magnification ×330). (Reprinted from Ref. 51; with permission from European Association of Feline Medicine.)
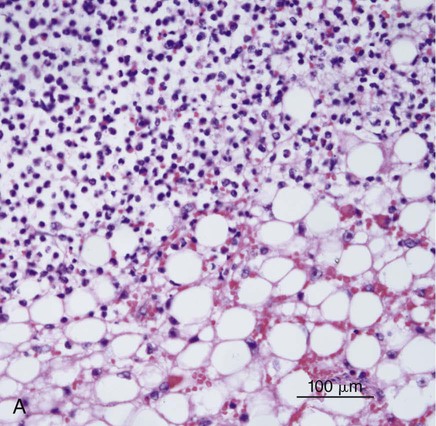
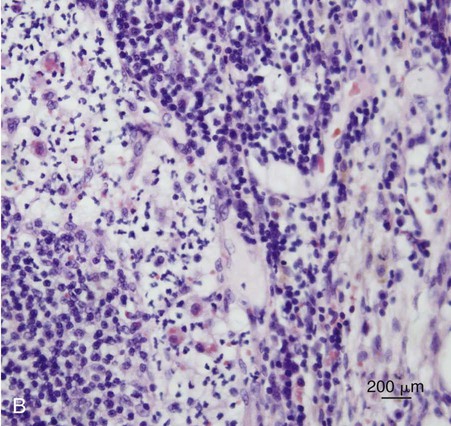
B, Histopathology of sternal lymph node from the same case. Note the neutrophilic exudate present in the cortical sinus. Involvement of the sternal lymph node was presumed secondary to the hepatitis observed in this case (H&E stain, bar = 200 µm). (Courtesy Mark Krockenberger, University of Sydney, Sydney, Australia.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


