Minimally Invasive Surgery
General Principles of Laparoscopy
Minimally invasive surgery (MIS) has become commonplace in both human and animal surgery. The benefits include smaller incisions, better visibility, less morbidity, and faster return to function.1,2 Minimally invasive techniques include thoracoscopy, laparoscopy, and arthroscopy. All minimally invasive techniques involve the use of special equipment and specialized training. Surgeons inexperienced in MIS techniques and having inadequate equipment may quickly become frustrated with the performance of these procedures. The quality of the light source, insufflation unit, and equipment are directly proportional to the success and satisfaction of the surgeon and the client.
Insufflation
Most laparoscopy is performed using carbon dioxide (CO2) insufflation to create a working space within the peritoneal cavity. CO2 has become the gas of choice, as it is rapidly absorbed from the abdomen, and gas emboli pose little risk to the patient.3 Delivery of CO2 into the abdomen is generally accomplished with a mechanical or electronic insufflator. The medical-grade gas is purchased in a compressed tank, hooked to the insufflator via a yoke, and delivered to the patient via a sterile tube. In human medicine, in-line, micropore filters are used to further limit the introduction of pathogens or irritants into the peritoneal space. The use of such filters in veterinary laparoscopy is variable, but are required if room air insufflators are used. The recent advent of natural orifice transluminal endoscopic surgery (NOTES) has raised the possibility of using compressed room air to perform surgery. In one large study on women with infertility issues, patients undergoing room-air pneumoperitoneum, compared with CO2 insufflation, were more likely to have wound infection (15.3% versus 2.7%, respectively) and abdominal discomfort (84.7% versus 6.9%, respectively).4 Despite these differences, the authors concluded that room-air pneumoperitoneum was safe, inexpensive, and easily available and could be used in low-resource settings. A more recent study comparing CO2 and room air in both laparoscopy and NOTES, found that room air was acceptable.5 Room-air insufflation is routinely used in field settings for laparoscopy in cattle.6
In humans and animals, the recommended intraabdominal pressure during laparoscopy is 10 to 15 millimeters of mercury (mm Hg), since hemodynamic changes are recognizable at higher pressures.7 If pressures are increased by greater than 20 mm Hg, compression of venous and arterial vessels produce a rise in systemic and pulmonary vascular resistance and a reduction in renal and mesenteric blood flow. Mean arterial blood pressure rises, and cardiac output diminishes proportionally. The physical stretching of the peritoneum may increase the vagal tone and trigger bradycardia. Peritoneal and splanchnic vessels may be mechanically compressed at high insufflation pressures, resulting in adhesions and peritonitis. In general, the intraabdominal pressure should be just enough to provide viewing of the areas of surgical interest but no more. When performing standing laparoscopy in camelids and horses, we recommend limiting intraabdominal pressures to 10 to 12 mm Hg or lower to keep the animals comfortable during the surgical procedure. With the animals in dorsal recumbency under general anesthesia, it is possible to use greater pressures. However, it should be noted that greater pressures, combined with the Trendelenberg position (head down, tail up) could create difficulty in ventilating the patient effectively. Mild intraabdominal insufflation pressures combined with the Trendelenberg position has been shown to be safe in llamas placed under general anesthesia.8 In dogs, the Trendelenberg position is rarely used; rather, the animals are tipped from side to side, and a ventral midline approach is used. This may be useful in camelids as well.9
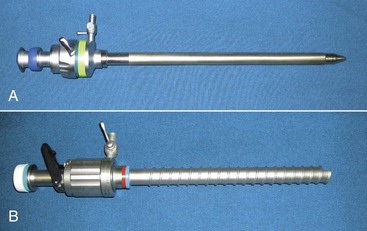
Insufflation may be delivered in the camelid species through a variety of methods, depending on the positioning of the animal. In standing animals, insufflation is most safely delivered through a 5- to 8-mm blunt-ended mare urinary catheter, a 5- to 10-mm laparoscopic cannula with a blunt obturator (Figure 64-1, A), or a controlled-access cannula (see Figure 64-1, B). Care should be taken when inserting the insufflation cannula to avoid penetrating the deeper structures or detaching the peritoneum causing a pneumoretroperitoneum. In dorsally recumbent animals, any of the standing techniques, along with using either a Veress needle or a teat cannula, may be used. In both instances, we prefer to use a 10-mm laparoscopic cannula with a blunt obturator.
Telescopes
Traditional telescopes used in camelid laparoscopy are 7 to 10 mm in diameter and 33 to 57 cm long. In camelid surgery, a shorter telescope is often adequate and, in fact, easier to manipulate than the long telescope generally recommended in equine laparoscopy. Most telescopes are Hopkins-type scopes with two channels, one for viewing lenses and one for a fiberoptic light supply. These telescopes are available in 0-degree or 30-degree forward-viewing models.10 The 0-degree telescopes are generally easier for the novice surgeon to manipulate within the abdomen, but the 30-degree telescopes provide a larger field of view for the surgeon. We prefer a 30-degree telescope for operative procedures. Operating telescopes are also available and add a third channel to introduce small-diameter instruments through the telescope. Operating telescopes allow the possibility of single-port surgery for exploration, aspiration, and small biopsy. Most instrument channels are between 3 and 5 mm in diameter and require specialized instruments.
Cannulas
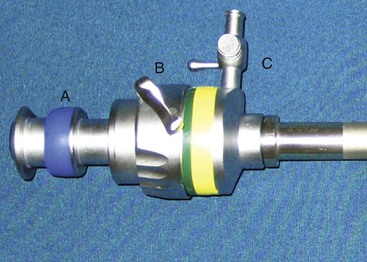
Cannulas have been developed to allow insertion of telescopes and instruments into the peritoneal cavity while maintaining adequate insufflation pressures. Cannulas come in a variety of diameters and are available in either disposable or reusable options. Generally, the smallest-diameter cannula is chosen on the basis of the diameter of the telescope and instruments. Cannulas are designed to have a two-gasket or two-valve system so that CO2 is not lost when instruments are being used through the cannulas or when the instruments have been removed from the cannulas (Figure 64-2). Most cannulas have a stop-cock on the main valve for the introduction of CO2 from the insufflator. The common shaft lengths of cannulas are 10, 15, and 20 centimeters (cm). In the majority of camelids, 10-cm-long cannulas are sufficient, and 20-cm-long cannulas (such as those used in equine flank laparoscopy) actually make the procedure more difficult. Recently, a cannula with an inflatable bulb and sliding lock-washer was described for laparoscopy in an alpaca.11 This offers the ability to maintain secure positioning of the cannula through the abdominal wall while minimizing the length of the cannula within the abdomen. This is particularly useful when multiple portals are needed and facilitates instrument usage without interference of one cannula with another. To insert the cannulas into the abdomen, an obturator is used. Reusable obturators are available in either blunt, conical, or pyramidal configurations. Disposable obturators are available as blunt, sharp, and guarded configurations. Blunt obturators are used for open approaches, whereas pyramidal or sharp obturators should only be used when direct visualization of cannula insertion is possible. This is important to prevent visceral trauma. Disposable cannulas or obturators offer the benefit of a guarded obturator that helps to reduce the possibility of bowel puncture during insertion. However, these cannulas are designed for single use, and their life span is limited, even though they can be reused after gas sterilization. We prefer to use reusable cannulas and obturators. These metal instruments are more resistant to breakage and long-term use.
Instruments
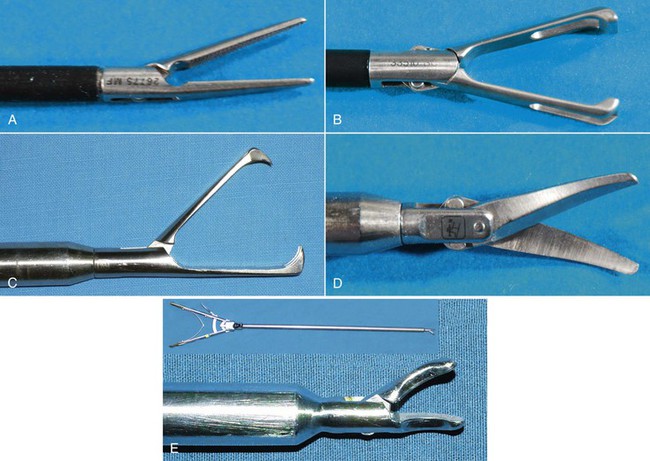
Essentially, all instruments that are available for open surgery have been redesigned for use in MIS. Most instruments available are either 5 or 10 mm in diameter and 33 to 45 cm in length. The most commonly used instruments in camelid laparoscopy are dissecting forceps (Figure 64-3, A), atraumatic grasping forceps (see Figure 64-3, B), traumatic grasping forceps (see Figure 64-3, C), scissors (see Figure 64-3, D), and needle holders (see Figure 64-3, E). Dissecting forceps and scissors are often available with a monopolar electrosurgical attachment for coagulation of small bleeders while performing surgery. (For more information, see the following section titled “Electrosurgical, Coagulating, and Vessel-Sealing Modalities.”)
Intracorporeal Techniques
Various techniques for intracorporeal MIS have been described. Endoscopic staples, endoscopic sutures, and endoscopic sealing devices have been increasingly available. The costs associated with the use of disposable staplers and suturing devices have limited the adoption of these instruments in veterinary MIS. Suturing in MIS is particularly challenging, and the surgeon should seek specific training in the use of these instruments and sutures prior to attempting these in clinical patients.12–14
Electrosurgical, Coagulating, and Vessel-Sealing Modalities15
Monopolar electrosurgery requires the use of an electrosurgical generator, an active electrode, and a grounding pad. Most of the time, monopolar electrosurgery is used in patients under general anesthesia. When used in a cutting mode, rather than in a coagulation mode, a lower wattage is used. Concerns with monopolar electrosurgery include poor contact of the grounding pad and aberrant and undetected energy delivery to other tissues through noninsulated structures such as cannulas and the telescope. Cutting and coagulating properties are both considered good. Monopolar electrosurgery is generally used in conjunction with another hemostatic modality such as a ligating loop. Tissue temperatures are often greater than 150°C. Vessels less than 3 mm in diameter may be successfully coagulated.
Bipolar electrosurgery uses an active electrode and a passive electrode similarly to monopolar electrosurgery; however, both electrodes are found in the tips of the instrument (Figure 64-4). Current passes only through the tissue that is found between the jaws of the instrument, reducing the possibilities of aberrant and undetected energy delivery to other tissues. Bipolar electrosurgery may also easily be used in standing sedated patients. Coagulation characteristics are good, but the cutting ability is poor, requiring either a separate application of scissors or the use of an integrated cutting blade in the bipolar forceps. When using an adequate generator, bipolar electrosurgery may be used for both ovariectomies and cryptorchidectomies. Tissue temperatures are often greater than 150°C. Vessels less than 3 mm in diameter may be successfully coagulated.
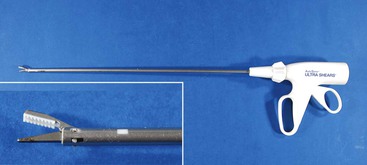
Use of radiofrequency devices is similar to bipolar electrosurgical modalities. The main difference is that the radiofrequency devices often incorporate “smart technology” to assist the surgeon and operate at higher frequencies (300 kilohertz [kHz] to 13 megahertz [MHz]). The most commonly used radiofrequency device in MIS is the LigaSure device, manufactured by Valley Lab (Valley Lab/Covidien, Boulder, CO). The LigaSure device is a feedback-controlled electrothermal sealer that applies a precise amount of energy to vessel walls to produce a denatured protein seal. Unlike the monopolar and bipolar electrosurgery modalities, which rely on a coagulum for coagulation, the LigaSure device seals the vessel walls together. The device measures the electrical impedance between its jaws and automatically shuts off when an appropriate seal has been generated. If an inappropriate grasping of tissue has occurred, the device does not work, signaling the user to regrasp. Tissue temperatures are kept below 150°C. It is approved to seal vessels up to 7 mm in diameter and can easily be used for both ovariectomy and cryptorchidectomy in camelids. It will not, however, seal the uterine body. Hand pieces with integrated cutting blades are available in both 5 and 10 mm diameters (Figure 64-5).

Figure 64-5 Picture of LigaSure hand instruments. A, 10-mm forceps. B, 5-mm forceps. Inset: Close-up of tips.
Ultrasonic cutting and coagulating devices are designed to use sound waves to provide the desired tissue effects. Sound waves may be delivered to cause tissue dissection, coagulation, and cutting. In most units, the blade system vibrates at a frequency of 55,500 cycles per second at a distance of 50 to 100 microns per cycle. The blade configuration determines the tissue effect; that is, sharper blades provide a better cutting effect and less coagulation compared with blunt blades (Figure 64-6). Some instruments are available with a jaw configuration in which one jaw holds the tissue stable while the other moves. As with radiofrequency, the tissue temperature is kept below 150°C. Vessels up to 3 mm in diameter have been successfully coagulated.
Patient Positioning
Standing laparoscopic ovariectomy has been reported in sedated llamas.16 The llamas were sedated with 0.1 milligram per kilogram (mg/kg) of butorphanol tartrate administered intravenously (IV) and placed in standing stocks. Standing cryptorchidectomy and general exploratory surgery are also options in llamas. Alpacas, however, tend to cush under stressful conditions and are probably not good candidates for standing surgery. The most common positioning for laparoscopic surgery in camelids is dorsal recumbency under general anesthesia. All reports at this time suggest placing the animal in the Trendelenberg position, which may require positive-pressure ventilation.
Laparoscopic Techniques in Camelids
At the time of writing this text, various techniques have been reported for MIS in camelids, including ovariectomy, ovariohysterectomy, cryptorchidectomy, abdominal exploratory surgery, cystotomy, and intraabdominal vasectomy.9,11,16–22 Other techniques that we have performed include organ biopsies and reestablishment of vaginal patency. NOTES may also be useful in the future of camelid MIS. This technique has been described in humans and uses natural orifices such as the stomach, vagina, or even rectum to access the peritoneal cavity.
Laparoscopic Ovariectomy and Ovariohysterectomy in Llamas and Alpacas
Camelids are induced ovulators and do not have a normal estrous period. Their follicles grow and regress in an overlapping wavelike pattern. Puberty generally occurs when the animals are between 10 and 12 months of age.23,24 Consequently, camelids can breed at almost any time after the onset of puberty. As these animals have taken on a greater role as companions, it has become more common to commingle males and females. If no offspring are desired, neutering of the male is an acceptable technique to stop reproduction. However, if the desire is to have selected females removed from the breeding population, ovariectomy is a viable technique. Indications might include congenital defects, difficulty in parturition, or other undesirable characteristics. Other pathologic indications of ovariectomy include follicular cysts, cystic corpora lutea, ovarian tumors, hydrosalpinx, ovarian abscesses, or paraovarian cysts.25–27 If pathologic conditions such as pyometra, mucometra, neoplasia, endometritis or segmental hypoplasia are present, ovariohysterectomy would be warranted.28
Pertinent Anatomy
The uterus in New World camelids is bicornuate. The cervix is approximately 2 to 5 cm long and 2 to 4 cm in diameter, whereas the uterine body is 2 to 4 cm in length and 2–4 cm in diameter, and the horns are 8.5 to 15 cm long.23,29 The left horn is generally larger than the right horn.
Standing Ovariectomy30
Patient Selection
Standing ovariectomy is more realistic in llamas than in alpacas, as they are more likely to stand throughout the procedure. Similar to standing equine laparoscopic ovariectomy, if the llama is difficult to handle, it is not a good candidate for standing surgery. This is a reasonable approach for well-behaved llamas and should be considered for removal of ovaries. Only ovariectomies in the standing animal have been reported. With appropriate equipment, removal of a moderate-sized uterus should also be possible (Box 64-1).