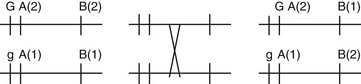
Chapter 221 The basis, methods, techniques, and quality control of inherited disease testing are not standardized for small animals. In addition, animal genetic testing laboratories and companies have no oversight or accreditation. Regulations and guidelines for small animal genetic testing are needed. Until these are enacted, veterinarians submitting samples for canine genetic disease testing must evaluate each test individually to determine its accuracy and reliability. A basic knowledge of the methods of the test and an evidence-based approach to its evaluation are important to guide decisions about when to use a specific test. Criteria for molecular genetic testing should be similar to those for any diagnostic medical test. Within a reasonable amount of time after development, the data and results of the test should be published in a peer-reviewed scientific journal. Ideally the test should be verified independently by an outside group. Any test, evidence, and data without the scrutiny of a peer review should be used cautiously or evaluated critically. For some of the currently available tests, only patent information is available; this information can be reviewed through the U.S. patent website (www.uspto.gov/main/patents.htm). In some cases companies offering tests are awaiting resolution of intellectual property issues. Biochemical tests for inherited disorders in dogs and cats have been available for many years. They continue to play an important role in diagnosis of inherited disorders in which the chromosomal location or gene for the defect has not been identified. Most biochemical tests require only a simple blood or urine sample. Biochemical tests are also necessary to help evaluate newly developed molecular genetic tests, especially those lacking documentation or presenting controversy. Examples of some of the currently available biochemical tests include those for mucopolysaccharidosis, Fanconi syndrome, erythrocyte osmotic fragility, methylmalonic aciduria, cystinuria, urinary acids, urinary amino acids, urinary carbohydrates, urinary glycosaminoglycans, urinary oligosaccharides, cobalamin malabsorption, hypersarcosinemia, and other inborn errors of metabolism performed at the University of Pennsylvania School of Veterinary Medicine (PennGen) (Table 221-1). Factor assay tests for von Willebrand’s disease and other inherited coagulopathies are performed at a number of veterinary diagnostic laboratories. In many cases biochemical tests are the best estimate of the genetic status of an individual (i.e., affected, carrier, or clear). However, test results can fall into overlapping categories, causing potential problems with classification and definition. TABLE 221-1 Some Laboratories and Companies Offering Canine and Feline Genetic Testing in the United States and United Kingdom A genetic marker can be associated strongly or linked with a disease gene if it is close to a gene and the marker has more than one allele. The farther a marker is from a gene on the same chromosome, the more likely recombination is to have occurred during meiosis. The percentage of time a marker and gene have recombination between them is termed the recombination fraction. For a marker to be potentially useful as a screening genetic test, a recombination fraction of 5% or less typically is required. Figure 221-1 illustrates marker and gene linkage and recombination. A marker allele is linked to a disease; this is probably the most difficult concept pertaining to an understanding of published chromosomal locations of causative genes and molecular genetic disease testing. Figure 221-1 Genetic linkage of a marker A and gene G and recombination between G and marker B. The left portion shows two homologous chromosomes with gene G and markers A and B. G refers to the normal gene allele and g refers to the mutated gene allele. A(2) is marker A allele 2, and A(1) is marker a allele 1. The left side shows the A(1) and B(1) marker alleles linked with the mutation g. The middle shows a recombination event during meiosis, and the right side shows that marker A, which is close to the gene, still has allele A(1) linked with the mutation g. However, marker B now has changed to allele B(2) linked with mutation g as a result of the recombination event. A marker must be close to the gene with a low recombination frequency and with specific population dynamics of the alleles to be a reliable screening test.
Methods and Availability of Tests for Hereditary Disorders of Dogs and Cats
Scientific Basis of the Tests
Biochemical Tests
Name
Phone
Website
Animal Health Trust (AHT) (United Kingdom)
44-(0)1638-555621
www.aht.org.uk
DDC Animal DNA Testing
800-625-0874
http://www.vetdnacenter.com/
OptiGen LLC (Ithaca, NY)
607-257-0301
www.optigen.com
Orthopedic Foundation for Animals (OFA) (Columbia, MO)
573-442-0418
www.offa.org/dnatesting/
PennGen (University of Pennsylvania)
215-898-3375
www.vet.upenn.edu/penngen
Veterinary Genetics Laboratory (University of California, Davis)
530-752-2211
www.vgl.ucdavis.edu
VetGen LLC (Ann Arbor, MI)
800-483-8436
www.vetgen.com
Deoxyribonucleic Acid–Based Tests
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


