Jeffrey Phillips
Melanoma
Melanomas are tumors that arise from the transformation of normal melanocytes, which are the pigment-producing cells not only in the skin but also throughout the body. Although these tumors occur naturally in all mammals, they are among the most common tumors seen in horses, comprising 3.8% to 15% of all skin tumors, and are second in frequency only to sarcoids. According to some studies, the incidence of these tumors in horses may be increasing in parallel with a reported increased incidence of the same tumor in humans. Although a gender predisposition has been suggested, no clear predilection has been established. In contrast, although melanomas have been diagnosed in all breeds and colors of horses, gray horses have a marked predisposition, with prevalence rates reaching as high as 80% in older animals.
Histologic Classifications
Melanocytic tumors in horses have been recognized for centuries as slowly growing but locally invasive tumors that frequently metastasize. The term melanocytic tumor encompasses all histologic and clinical variants, from the benign melanocytoma (nevus) to the anaplastic malignant variants. In nongray horses, melanocytic tumors include only benign (melanocytoma) and malignant variants. In gray horses, however, there seems to be a clinical continuum between benign and malignant tumors, and the melanocytic disease process is further extended to include hyperpigmentation and infiltration of the dermis and epidermis that results in development of plaquelike lesions rather than true masses or tumors.
Histologically, neoplastic melanocytes are described as mildly to moderately pleomorphic epithelioid to spindle-shaped cells with euchromatic nuclei. Cells may rarely be binucleate, have variable and often high cytoplasmic pigmentation, and have occasional mitotic figures. The tumors themselves are classified into distinct histologic subtypes on the basis of tumor cell morphology and location in the cutaneous adnexa. Benign-appearing collections of melanocytes in the superficial dermis or dermoepidermal junction are classified as melanocytomas (melanocytic nevi). Tumors in deep dermal locations and composed of well-differentiated melanocytes that have dense cytoplasmic pigmentation and minimal malignant criteria are classified as dermal melanomas. Dermal melanomas are further subdivided clinically into tumors composed of a few discrete masses or nodules and a more disseminated variant with multiple, frequently confluent, tumors (dermal melanomatosis). An alternate descriptive classification relies only on tumor cell morphologic features and traditional malignancy criteria to group tumors into either benign or malignant variants. Benign variants contain well-differentiated and heavily pigmented melanocytes. These benign tumors are often contained within a pseudocapsule, and the cells can have a variable mitotic index. Malignant tumors are characterized by increased pleomorphism, variable pigmentation, moderate to high mitotic rates, evidence of vascular or lymphatic invasion, epidermal invasion, and indistinct tumor margins.
Molecular Genetics
Studies have been undertaken to elucidate the molecular basis of equine melanoma as a comparative model for human melanocytic tumors. Most researchers have focused on gray horses because of their increased risk for tumor formation associated with the loss of coat color secondary to graying. Recent work has identified the genetic basis for this premature graying as a 4.6-kb duplication in intron 6 of the STX17 gene, which leads to the overexpression of STX17 and the neighboring gene NR4A3. This duplication also appears to contain regulatory elements that have melanocyte-specific effects. These effects transform a weak enhancer to a strong melanocyte-specific enhancer, each of which encodes binding sites for microphthalmia-associated transcription factor. Microphthalmia-associated transcription factor regulates melanocyte development, and these binding sites within the STX17 gene provide a plausible explanation for the melanocyte-specific effects of the Gray allele, including hair graying, melanoma susceptibility, and vitiligo. Although STX17 is inherited in an autosomal dominant fashion, the risk for melanocytic tumor formation and the other traits associated with this mutation appear to be polygenic.
Mutations in melanocortin-1 receptor (MC1R) signaling have also been studied to determine their role in melanocytic tumor development. Specifically, a single nucleotide polymorphism in MC1R (C901T) has been linked to chestnut coat color and a resultant low risk for melanocytic tumor development. A loss of function mutation (ADEx2) in the agouti signaling protein (ASIP), a known antagonist of MC1R, has been linked to black coat color and an increased risk for melanoma formation. In addition to the upregulation of downstream genes such as tyrosinase, enhanced signaling through the MC1R pathway also results in markedly increased expression of the NR4A nuclear receptor subgroup in melanocytic cells. As mentioned, overexpression of NR4A3 has been found in gray horse melanomas, although it has not been directly associated with the development of melanocytic tumors in humans or horses.
The molecular basis for malignant transformation of melanocytic tumors has also been investigated. Copy number expansion of the STX17 duplication has been identified in tumor tissue of gray horse melanoma, and it has been speculated that increasing copy number may be associated with tumor aggressiveness. RACK1 serves as an anchoring protein for protein kinase C, and in this role likely plays a vital role in cellular signaling. The RACK1 protein has also been associated with melanocytic tumor transformation. Immunofluorescence detection of RACK1 appears to be useful for differentiating benign and malignant melanocytic tumors.
Diagnosis and Treatment Options
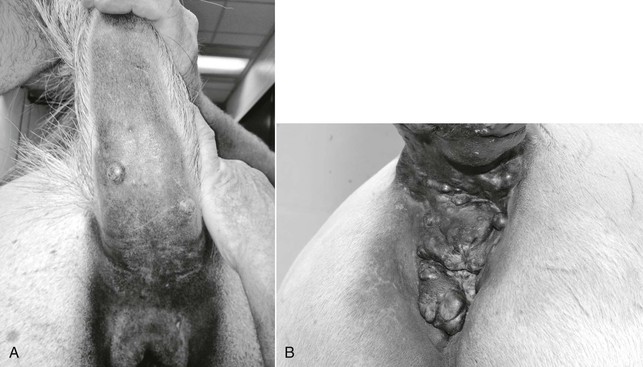
Tumors in affected horses are often located in the perineal region, under the tail, along the ventrum or extremities, on the prepuce, on the head or neck, or in visceral locations, with metastases commonly noted at other cutaneous sites, lymph nodes, and viscera (Figure 125-1). Melanocytic tumors are generally heavily pigmented; however, areas of depigmentation or vitiligo can be identified within tumor tissue. Further, amelanotic or poorly pigmented tumors occur in both gray and nongray horses. Tumors can be localized in the deeper dermal tissues or may involve more superficial dermis and epidermal tissue. Tumors that involve superficial tissues often ulcerate through the epidermis as they enlarge. Progressive tumor enlargement can also result in central portions becoming necrotic as the blood supply is outgrown.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


