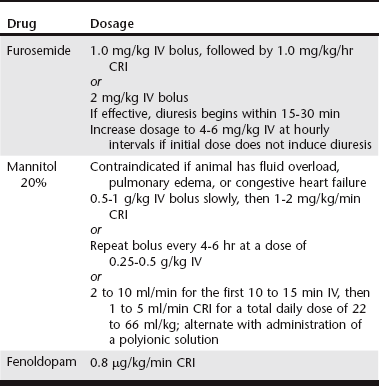
Chapter 191 The term acute kidney injury (AKI) has replaced the historical term acute renal failure because it is believed to better describe the pathophysiologic changes and duration of the different phases of injury. It is defined as the rapid loss of nephron function (over hours to several days) resulting in azotemia; fluid, electrolyte, and acid-base abnormalities; and uremia. Many causes of AKI have been identified in dogs and cats. Although supportive therapy is similar in all cases, the prognosis and clinical outcome have been shown to vary depending on the cause (Vaden et al, 1997). Intravenous fluid therapy remains the mainstay of treatment for AKI. Frequent monitoring of the animal’s hydration status, renal function, acid-base status, and electrolyte levels is necessary to determine appropriate intravenous fluid types and amounts. Placement of a catheter in the jugular vein allows monitoring of central venous pressure and more precise assessment of intravascular volume status. However, if hemodialysis is a treatment option, the jugular veins should not be used for intravenous catheters or even for venipuncture to obtain blood samples; rather, they should be preserved for placement of a central catheter for hemodialysis or other renal replacement therapy (see Chapter 192). The initial volume of fluid to be administered should be calculated based on the animal’s body weight and degree of hydration. Water deficits should be corrected within 4 to 6 hours to restore renal blood flow to normal as soon as possible. Maintenance fluid requirements (44 to 66 ml/kg/day) must be met and estimated fluid losses from vomiting or diarrhea replaced. Urine production should be monitored during the first few hours of fluid therapy. Placement of an indwelling urinary catheter is the most accurate method for such monitoring. However, the benefits of an indwelling catheter must be weighed against the risk of ascending infection and the need for sedation or anesthesia to place the catheter. The risk of infection can be reduced by scrupulous attention to sterile placement of the catheter, maintenance of a closed collection system, and daily cleansing of the visible portions of the catheter with disinfectant. Changing the urinary catheter every 2 to 3 days is recommended because the incidence of catheter-induced infections increases rapidly after 3 days when the same catheter is left in (Barsanti, 2010). Traditionally, intravenous fluids have been administered at as high a rate as the animal will tolerate without adverse signs, with the goal of maximizing glomerular filtration rate and renal blood flow and increasing elimination of metabolic waste products. However, an increase in fluid administration does not necessarily equate to increased urinary excretion of such substances. Recent studies in people have concluded that fluid overload is associated with adverse consequences and decreased survival; mortality decreased when fluid overload was corrected by dialysis (Bouchard et al, 2009). Although similar studies in clinical veterinary patients have not been reported, it would seem reasonable that avoiding fluid overload would be similarly beneficial, especially because dialysis is not readily available to many practices. One of the reasons for fluid overload is failure to adjust the fluid administration rate in the face of decreased urine production (see Chapter 186). Specific therapy to increase urine flow, consisting of administration of one or more diuretics, should be instituted next (Table 191-1). Furosemide can be administered as a bolus of 2 mg/kg IV, with escalation of doses to 4 to 6 mg/kg at hourly intervals if the initial dose fails to increase urine production. However, constant-rate infusion (CRI) has been shown to be more effective in producing diuresis than intermittent bolus doses (Adin et al, 2003). A loading dose of 1.0 mg/kg followed by a CRI at 1.0 mg/kg/hr is recommended. If furosemide administration fails to increase urine flow, osmotic diuresis can be attempted. Twenty percent mannitol can be given as a bolus dose of 0.5 to 1 g/kg of body weight over 15 to 20 minutes. If it is effective, urine flow will increase within 1 hour. Repeat bolus doses then can be administered every 4 to 6 hours, or mannitol can be administered as a CRI at 1 to 2 mg/kg/min. Mannitol may have additional beneficial effects in addition to its action as a diuretic. It inhibits renin release because of its hyperosmolar effect on tubular luminal filtrate. Mannitol also acts as a free-radical scavenger, blunting damaging increases in intramitochondrial calcium, and may result in a beneficial release of atrial natriuretic peptide. In actuality, mannitol is used infrequently because administration of a hypertonic solution is contraindicated in oliguric animals that are volume overloaded, and oliguria often is not recognized until overload already is present. Dopamine infusion traditionally has been recommended for oliguric or anuric animals. However, it is no longer considered to have a role in the prevention or treatment of AKI in people, based on several metaanalyses that failed to show a clinical benefit with regard to survival or need for dialysis (Friedrich et al, 2005; Kellum and Decker, 2001). There is little or no documentation of the efficacy of dopamine in dogs and cats with AKI, and its routine use to increase urine production in oliguric or anuric AKI cannot be justified.
Medical Management of Acute Kidney Injury
Supportive Therapy
Fluid Therapy
Management of Oliguria or Anuria
< div class='tao-gold-member'>
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Medical Management of Acute Kidney Injury
Only gold members can continue reading. Log In or Register to continue