CHAPTER 4 This chapter will also discuss and illustrate selected “especially dangerous and contagious microorganisms” because diseases caused by these pathogens can have disastrous impact on livestock health and production and on the economies of affected countries. The location in this textbook of coverage of these diseases considered by the USDA/APHIS and the World Organization for Animal Health (OIE) as “Foreign Animal Diseases” or “OIE Reportable Diseases” is listed in Table 4-1. TABLE 4-1 Location in this Textbook of the Coverage of Especially Dangerous and Contagious Microorganisms Infectious microorganisms follow chronologic sequences of events regulated by virulence determinates to infect target cells unique to specific organ systems and cause disease (Fig. 4-1). They most commonly enter the body through ingestion, inhalation, or cutaneous penetration and interact with mucosae or skin. If their target cell is not in mucosae or skin, they may spread to submucosal and subcutaneous lymphoid nodules such as in tonsils or Peyer’s patches, then to regional lymph nodes, and then systemically in the circulatory and/or lymphatic systems to other organ systems. They often infect macrophages, lymphocytes, and/or dendritic cells and use these cells to spread via leukocyte trafficking to target cells in organ systems as they migrate through these systems as part of their normal immunologic surveillance activities. Cell and tissue specificity is based on ligand-receptor interactions in which proteins (ligands) expressed on the surface of infectious organisms bind to receptors on membranes of host target cells, mucus associated with these cells, vascularized extracellular matrix (ECM) tissues beneath these cells, or macrophages, lymphocytes, and dendritic cells supporting these cells. Once bound to receptors, a sequence of events facilitated by virulence determinates is initiated that colonizes the surface of these cells or invades the cells through phagocytosis or endocytosis. The organism then establishes control of normal metabolic systems of these cells and uses them to replicate in and spread to other organ systems. The outcome of this process is usually cell dysfunction and/or death and thus clinical disease. Fig. 4-1 Sequence of events in infection. Except for contact with carrier animals, the chronologic sequence of events leading to disease caused by infectious organisms are not random events. Contact places susceptible animals in close proximity to infected animals, where infectious organisms can be spread through direct contact, grooming, licking, bite wounds, sneezing, and other normal physiologic body processes in fomites from body fluids, water droplets, snot, sputum, urine, and feces. Infection depends on creating an initial beachhead to establish, sustain, and spread the infection when organisms first encounter tissues of the body. Generally, the initial beachhead is established in one of two tissue types: (1) mucosae of the respiratory system (nasal cavity, nasal pharynx, conductive system [see Chapter 9]), alimentary (small intestine) system (see Chapter 7), lower urinary system (see Chapter 11), reproductive systems (see Chapters 18 and 19), or ear and eye (conjunctiva) (see Chapter 20) or (2) subcutaneous tissues, including muscle and endothelial cells of the skin (Fig. 4-2). In these beachheads, organisms gain access and attach to, enter, and/or replicate in mucosal- and connective tissue-associated macrophages, lymphocytes, and dendritic cells. It is from these beachheads that they then spread locally (submucosae and subcutis), regionally (lymph nodes), and/or systemically (organ systems) to other target cells to sustain and amplify the infection and cause disease (Fig. 4-3). Fig. 4-2 Portals of entry. Fig. 4-3 Mechanisms of Microbial Infections and Pathways of Spread. A concept central to the pathogenesis of disease is the ability of an infectious organism to reach a site in the body suitable for growth and replication. Ingestion, inhalation, cutaneous penetration, and ascending infection are the most common portals of entry for infectious organisms to gain access to mucosae of the respiratory and alimentary systems; the epidermis, dermis, and subcutis of the integumentary system; and the lower urinary system and reproductive systems (see Fig. 4-2). The mucosae of the alimentary and respiratory systems are covered by a protective mucus gel composed predominantly of mucin glycoproteins synthesized and secreted by goblet cells (Fig. 4-4). The mucus layer forms a barrier system that attempts to do the following: Fig. 4-4 Mucus layer of alimentary and respiratory mucosae. 1. Block infectious organisms from reaching target cells. 2. Trap infectious organisms so they can be phagocytosed by mucosal macrophages and neutrophils. 3. Trap infectious organisms so they can be exposed to bacteriostatic and bacteriocidal molecules sequestered in the mucin matrix. 4. Facilitate phagocytosis of infectious organisms via mucosa-associated macrophages, mucosal dendritic cells, and microfold (M) cells. 5. Deliver microbial antigens to local lymphoid tissues like Peyer’s patches or bronchial-associated lymphoid tissues (BALT) and then to regional lymph nodes via afferent lymphatic drainage. Organisms enter the alimentary system (see Chapter 7) by ingestion of infectious fomites. Through the processes of mastication, swallowing, and peristalsis, infectious organisms gain access to and are trapped in the mucus layer of the oral pharynx and intestines. Mucosae most commonly involved include tonsillar epithelium, villus epithelium, crypt epithelium, and epithelium containing M cells overlying Peyer’s patches. Infectious organisms then must penetrate the mucus layer to reach targets such as mucosal epithelial cells, dendritic cells, and tissue macrophages. M cells are also targets but lack a mucus covering. Mucus in the alimentary system is produced by goblet cells distributed among mucosal epithelial cells in the villi and crypts where it covers and protects microvilli (see Fig. 4-4). The mucus layer is a (1) physical and (2) biologic barrier protecting the intestine against infectious organisms via (1) its thickness and viscosity, (2) binding to bacterial adhesins, (3) serving as a reservoir for immunoglobulin A (IgA) and lysozyme, and (4) acting as a free radical scavenger. Additionally, the mucus layer is a habitat for beneficial enteric microflora. Generally, there are more goblet cells in the large intestine than the small intestine, more in crypts than in villi, and more in the ileum than in the jejunum or duodenum. It appears that mucus covers all intestinal epithelial surfaces to varying degrees of thickness and viscosity and is composed of an inner gel layer and an outer soluble layer. The mucus layer is thickest in the colon (≈830 µm) and thinnest in the jejunum (≈123 µm). It is less prominent over absorptive enterocytes with microvilli when compared to crypt enterocytes. M cells are not covered by a mucus layer; therefore infectious organisms can readily interact with their cell membranes. Once entrapped in the mucus layer, infectious organisms must then penetrate it to gain access to target cells for infection. Additionally, infectious organisms also encounter mucosal fluids, such as gastric acid, mucins, secretions such as lysozyme, and humoral mediators such as immunoglobulins, and also compete with normal microflora for resources. Mucosae-associated lymphoid tissues (MALT), such as Peyer’s patches (Fig. 4-5), are submucosal lymphoid nodules located in the distal jejunum and ileum that surround groups of intestinal crypts. Nodules are composed of lymphocytes, macrophages, and dendritic cells. They are covered by modified epithelial cells of intestinal crypts called M cells that transfer antigens in the lumen of the intestine across the mucosa to dendritic cells and immune cells like T lymphocytes in the nodule. M cells are the interface between materials in the lumen of intestinal crypts and the lymphoid nodules (see Fig. 4-5). Peyer’s patches have afferent lymphatic vessels that drain to regional mesenteric lymph nodes. Fig. 4-5 Microbial interactions with a barrier system: intestinal mucosa. In the respiratory system (see Chapter 9), infectious organisms are inhaled through the nostrils and are deposited on mucosae of the nasal turbinates, nasal pharynx, and/or the conductive system based on physical properties of the agent such as size, shape, weight, and electrostatic charge (Fig. 4-6). Groups of infectious organisms ranked from smallest to largest include: viruses (5 to 300 nm [1 × 10−9 m] in diameter), prions (≈16 nm in diameter), bacteria (0.5 to 5 µm [1 × 10−6 m] in diameter), fungi 5 to 60 µm in diameter, and protozoa 1 to 300 µm in diameter. Although it is convenient to compare infectious organisms based on size, rarely are these organisms inhaled as free infectious organisms. Most commonly, they are enclosed in fomites (i.e., inanimate objects or substances capable of carrying infectious organisms) such as dust particles, soil, septum, or body fluids. Thus the physical properties of infectious fomites determine where they are deposited on mucosal surfaces of the respiratory system and cause disease. When inhaled, larger infectious fomites, such as bacteria and fungi, are deposited and trapped in the nasal turbinates, whereas in a gradient of descending size, smaller ones are able to reach the pharynx, larynx, trachea, and bronchi before they are deposited and trapped in the mucosae. The nasal cavity and turbinates trap 70% to 80% of particulate matter 3 to 5 µm or greater in diameter; 60% of particulate matter 2 µm or greater but cannot trap particles with sizes <1 µm in diameter. In a normal functioning respiratory system, only infectious fomites of 1 µm or less in diameter, such as viruses and some bacteria, can be inhaled into bronchioles, alveolar ducts, and alveoli, which are the oxygen-carbon dioxide (O2-CO2) exchange portion of the respiratory system. Fig. 4-6 Deposition of infectious microorganisms. When infectious fomites are inhaled, they first encounter the nasal turbinates. The movement of air through the turbinates causes centrifugal turbulence that forces them against the mucosae where they are trapped in its overlying mucus layer for removal. If the size of fomites allows them to pass through the turbinates and be carried to the pharynx, larynx, trachea, or bronchi, inertial turbulence forces fomites against airway mucosae where they are trapped in the mucus layer for removal. Inertial turbulence occurs when a laminar stream of airflow is disrupted by a septum within the conductive portion of the respiratory system when airways branch. When the flow is split by a septum, the flow rotates centrifugally on either side of the septum and forces fomites against the mucosae. Depending on the species, airways can branch 23 times en route from the trachea to the alveoli. The O2-CO2 exchange portion of the respiratory system (the bronchioles, alveolar ducts, and alveoli) are not ciliated and have no protective mucus layer because of their gas exchange function. The outcome of these turbulence mechanisms is to trap infectious fomites in the mucus layer overlying ciliated mucosal epithelial cells. When infectious fomites are trapped in the mucus layer, they are (1) acted on by other components of the innate immune system, such as phagocytes like alveolar macrophages and neutrophils, and microbicidal molecules, such as lysozyme and immunoglobulins, and (2) removed by the mucociliary apparatus (see Chapter 9). The mucociliary apparatus is composed of the mucus layer and ciliated mucosal epithelial cells and is an important defensive mechanism in the respiratory system (Fig. 4-7). The mucus layer, produced by goblet cells and submucosal glands, is biphasic and consists of a luminal viscoelastic or gel layer used to trap infectious fomites and a serous inner layer in which the cilia of ciliated mucosal epithelial cells beat (see Fig. 4-7). The tips of the cilia just slightly enter the gel layer and their beating moves the gel and fomites. In the nasal cavity and sinuses, cilia move mucus and debris downward toward the pharynx for swallowing; in the conductive portion of the respiratory system, cilia move mucus and debris upward toward the pharynx for swallowing. The directionality of mucus flow is determined by the rhythmic unidirectional beating pattern of the cilia. The conductive portion of the respiratory system has an anterior and ventral flow pattern of distribution such that gravity influences the deposition of infectious fomites, if the mucus layer and/or the mucociliary apparatus are dysfunctional. Therefore injury to mucosal epithelial cells by specific infectious organisms, such as influenza and bovine rhinotracheitis viruses, can disrupt the function of the mucociliary apparatus, thereby exacerbating an existing disease or creating an opportunity for a secondary microbial infection of dependent lung usually prevented by this clearance mechanism. Swallowing of infectious organism-infected mucus may be a mechanism to clear certain bacteria; however, it provides other bacteria, such as Rhodococcus equi, the opportunity to gain access to the alimentary system and cause disease. The mucosae of the conductive portion of the respiratory system also contain dendritic cells and alveolar and tissue macrophages that commonly migrate through the mucosae and the mucus layer during their normal patterns of leukocyte trafficking (see Chapters 5 and 13). Because these cells can phagocytose and kill infectious organisms, they serve as a primary defense mechanism against infections. However, certain infectious organisms have virulence determinates that allow them to evade killing by phagocytes and use them like a “Trojan horse” to spread the agent and infect other cells and tissues. These cells are common targets for infectious organisms and along with mucosal epithelia serve as the initial beachhead infection before organisms spread locally often to the tonsil, regionally to lymph nodes, and systemically to other organ systems. bronchial-associated lymphoid tissues (BALTs) are submucosal lymphoid nodules located subjacent to ciliated mucosae, usually in areas in which inertial turbulence deposits foreign material on mucosae (see Fig. 4-5). Nodules are composed of lymphocytes, macrophages, and dendritic cells and function much like Peyer’s patches. BALTs have afferent lymphatic vessels that drain to regional tracheobronchial lymph nodes. Fig. 4-7 Mucociliary apparatus. Infectious organisms enter the skin, dermis, and subcutaneous tissues (see Chapter 17) via penetration through abrasions, scratches, and bite wounds or through bites of insect vectors such as mosquitoes that spread the agent into subcutaneous tissues such as muscle, blood vessels, and connective tissue (see Fig. 4-2). In these tissues, organisms encounter a limited range of host target cells such as epithelial cells in the skin, dendritic cells (Langerhans’ cells), tissue macrophages, endothelial cells of the vascular system, and connective tissues and muscle of the dermis and subcutis. They may also be deposited directly in the blood vascular system via penetration of a capillary or venule by an insect proboscis. Additionally, organisms encounter body fluids, such as blood and plasma proteins, that serve as resources for survival, infection, and replication. Mucosal and cutaneous epithelia of the respiratory, alimentary, integumentary, lower urinary, and reproductive systems are the interface between the outside and inside of the body and are held together by occluding junctions such as tight junctions, desmosomes, and adherens junctions and to the basement membrane and ECM by anchoring junctions. Thus these cells form barrier systems that protect the body against entry by infectious organisms. In the respiratory and alimentary systems (and likely in other mucosae), the surface of an epithelial cell located above its junctional complexes and exposed to the lumen is called the apical domain, whereas the surface below junctional complexes on the sides and base make up the basolateral domain (Fig. 4-8). This relationship establishes a polarity to the cell, and it has been shown experimentally that such polarity is often reflected in the expression of different sets of cell membrane receptors that can potentially be used by infectious organisms to attach to and enter the cells. As examples, parvoviruses use receptors expressed only on the basolateral surfaces to infect small intestinal crypt cells via Peyer’s patches, whereas influenza viruses use receptors expressed only on apical surfaces to infect respiratory epithelial cells via the airway. Infectious organisms enter cells through a process called receptor-mediated endocytosis most commonly at the apical surface and exit the cell from the basolateral surface via a mechanism called exocytosis. Fig. 4-8 Domains of polarized epithelial cells in mucosal barriers. The innate and adaptive immune responses are covered in detail in Chapters 3 and 5. The monocyte-macrophage system is covered in detail in Chapter 5. In summary, cells of the monocyte-macrophage system form a network of phagocytic and immune cells like alveolar macrophages, mucosal macrophages, and tissue macrophages (Fig. 4-9) that migrate throughout the body in the circulatory and lymphatic systems looking for foreign matter and infectious organisms and return to local, regional, and systemic lymphoid tissues to share antigens with resident immune effector cells. These cells are important in phagocytizing and killing infectious organisms (Fig. 4-10) and commonly encounter infectious organisms, such as bacteria and viruses, in mucosae and the mucus layer. They migrate through the mucus layer, phagocytizing infectious organisms and material they recognize as foreign and carry it via cell migration through the mucosal epithelium to local lymphoid tissue using a mechanism called leukocyte trafficking (Fig. 4-11). Local lymphoid tissues, such as Peyer’s patches and BALT, contain lymphocytes and macrophages that can be infected with organisms present in trafficking macrophages (see Fig. 4-5). From these local sites, infected lymphocytes and macrophages spread via lymphatic vessels to regional lymph nodes where additional cells are infected and then spread via lymphatic vessels to the thoracic duct and the circulatory system. From here, infected cells spread systemically to other organ systems in which specific target cells are infected, including lymphoid organs such as the spleen, lymph nodes, and bone marrow. Under normal conditions, tissue macrophages are derived from two sources: blood monocytes and tissue macrophage progenitor cells that are distributed throughout body tissues during organogenesis of the embryo. Precursor monocytes in bone marrow are capable of providing monocytes that migrate to and differentiate into macrophages in tissues. Tissue macrophages are also replenished locally and in large numbers by proliferation of tissue macrophage progenitor cells. These two populations of cells give rise to tissue macrophages that form the functional basis for innate and adaptive responses to microbes in tissues and organs (see Chapters 5 and 13). Tissues and organs that utilize cells of the monocyte-macrophage system include (1) lung (alveolar macrophages), (2) liver sinusoids (Kupffer cells), (3) lymph nodes (free and fixed macrophages), (4) spleen (free and fixed macrophages), (5) bone marrow (fixed macrophages), (6) connective tissue (histiocytes), (7) serous fluids (pleural and peritoneal macrophages), and (8) skin (histiocytes, Langerhans’ cells) (see Fig. 4-9). Fig. 4-10 Phagocytosis and intracellular destruction of microbes. Fig. 4-11 Leukocyte trafficking. Dendritic cells are covered in detail in Chapters 3 and 5. In summary, dendritic cells are migratory phagocytic antigen-processing and -presenting cells of mucosae (see Fig. 4-5) and skin (e.g., Langerhans’ cells) that are commonly found intermixed with epithelial cells. Infected dendritic cells can migrate from mucosae and skin to local and regional lymphoid tissues via lymphatic vessels. Infectious organisms through their surface proteins are able to bind to receptors expressed on the apical domains of these cells and infect them, then exit via the basolateral domain via exocytosis, gain access to lymphoid nodules (tissues commonly associated with dendritic cells), and establish a local infection in lymphocytes and macrophages. Infected lymphocytes and macrophages spread the agent via leukocyte trafficking from local sites to regional lymph nodes and then systemically to other organ systems as discussed for the monocyte-macrophage system. The resistance of animals to disease depends on the effective interplay of many structural and functional (physiologic) components of the body, including cutaneous and mucosal barrier systems and the immune system, respectively. Distinct networks of genes play central roles in structural and functional activities of the body. They control the development, maturation, and maintenance of epithelial cells, mucus, and supporting ECM tissues, such as collagen, that form the barrier systems. Additionally, they control similar structural activities for a variety of cell lineages of the innate and adaptive immune systems like T lymphocytes, macrophages, neutrophils, and dendritic cells and the expression of proteins that form pattern recognition receptors in the membranes of these cells (see Chapter 5). These receptors recognize pathogen-associated molecular patterns (PAMPs) expressed by infectious organisms and are discussed in greater detail in Chapters 3 and 5. Genes also play a central role in functional processes of cells, including adhesion, chemotaxis, phagocytosis, phagosome-lysosome fusion, intracellular killing of microbes, and antigen processing (see Chapters 3 and 5) involved in innate and adaptive responses of the immune system. Thus genetic resistance to infectious diseases is a polygenic trait regulated mainly by the immune system and its interactions with barrier systems and environmental factors such as weather conditions and nutritional status. An example of a genetic disorder that predisposes animals to infectious disease that occurs because of an alteration in the development of the basic structure of a barrier is epitheliogenesis imperfecta. It is an autosomal recessive hereditary disease of young horses, cattle, and pigs characterized by loss of epithelium affecting the skin and mucosae of the oral cavity and tongue likely caused by alterations in the subbasal plate and its hemidesmosomes and laminin-5 (see Chapter 17). Loss of the skin or mucosae exposes underlying vascularized ECM tissues to environmental contamination with feces and other matter allowing bacterial pathogens access to matrix tissues and capillary beds. The innate immune system provides animals with an immediate defense against infectious organisms and is discussed in detail in Chapters 3 and 5. In summary, this system involves the initial encounter with infectious organisms often at the barriers formed by mucosae and skin and the fluidic and cellular processes that ensue (see Chapter 3). It is essentially acute inflammation and the cellular and chemical mediators associated with the process, such as phagocytic cells like macrophages, neutrophils, and dendritic cells; effector cells, such as T lymphocytes and mast cells; chemical mediators of the complement system; and the vascular system. The purpose of acute inflammation is to dilute and isolate infectious organisms in edema fluid and fibrin, phagocytose and kill them, and process and present their antigens to effector cells of the adaptive immune response. When epithelial cells, endothelial cells, or mucosal or cutaneous macrophages of barrier systems are injured by or infected with infectious organisms, they release large quantities of cytokines into the surrounding tissues. These cytokines recruit, via chemotaxis, inflammatory cells from capillaries in vascularized ECM tissues and cause vasodilation and increased permeability of these blood vessels (i.e., edema fluid and fibrin). Inflammatory cells also release cytokines and other chemical mediators that act to recruit additional inflammatory cells, activate the complement cascade to identify bacteria and kill infectious organisms, promote phagocytosis of dead cells and infectious organisms by phagocytic cells, and activate the adaptive immune system through antigen processing and presentation to immune cells such as T and B lymphocytes. Phagocytic and effector cells of the acute inflammatory response are recruited from capillaries and migrate along a chemoattractant gradient formed by chemical mediators and molecules released from infectious organisms to the inflammatory focus. In inflammatory foci, these cells express Toll-like receptors (TLRs), also known as pattern-recognition receptors (PRRs) that recognize molecules on infectious agents called PAMPs (see Chapters 3 and 5) (Fig. 4-12). These cells also express interleukin-1 (IL-1) receptors that act in concert with PRRs to initiate and sustain the innate immune response through phagocytosis. Fig. 4-12 Toll-like receptors in the process of phagocytosis. Genetic disorders can affect all of the steps involved in the innate immune response from initial recognition of infectious organisms to their phagocytosis and killing and are discussed in many chapters of this book. Examples of genetic disorders of the innate immune system that predispose animals to infectious disease, most commonly bacterial diseases, include leukocyte adhesion deficiencies and granulocytopathy syndromes. Leukocyte adhesion deficiency occurs in dogs and cattle and has an autosomal recessive mode of inheritance. It is characterized by alterations in the leukocyte adhesion cascade (see Chapter 3) involving deficiencies or dysfunction of integrins and selectins resulting in the inability of neutrophils to adhere to endothelial cells in the wall of blood vessels and migrate into sites of bacterial infection. Granulocytopathy syndrome occurs in dogs and cattle and has an autosomal recessive mode of inheritance. It is characterized by alterations in the ability of neutrophils to kill bacteria in phagosomes and is linked to reduced nicotinamide adenine dinucleotide phosphate (NADPH) concentrations that may arise from a metabolic anomaly in the hexose monophosphate shunt (see Fig. 4-10). This deficiency may lead to reduced concentrations of hydrogen peroxide in phagosome-lysosome fusion and the bactericidal capability of the neutrophil. The process of phagocytosis is usually normal. Affected animals have shortened lifespan, long-term febrile disease, dermatitis, oral ulcers, lymphadenitis, and poor healing, attributable to irresolvable and repeated bacterial infections. Genetic disorders of the adaptive immune response are disorders in which affected animals are incapable of generating antigen-specific immune responses (see Chapter 5). Such genetic diseases are closely associated with genes that regulate the expression of the MHC, especially those genes involved in antigen processing and presentation. Examples of this type of disorder include agammaglobulinemia (a B lymphocyte immunodeficiency) and severe combined immunodeficiency (a B and T lymphocyte immunodeficiency). T lymphocyte, macrophage, and complement immunodeficiencies also occur but are not discussed here. Agammaglobulinemia has a X-linked recessive mode of inheritance and thus is a disorder of young colts. It is likely caused by dysfunction of cytoplasmic tyrosine kinase resulting in blockage in the differentiation of B lymphocyte lineages and a nearly complete absence of B lymphocytes and plasma cells. Clinically, this type of immunodeficiency results in colts with chronic bacterial diseases leading to pneumonia, enteritis, dermatitis, arthritis, and laminitis. Severe combined immunodeficiency occurs in dogs and Arabian horses and has an autosomal recessive mode of inheritance. In dogs, an X chromosome–linked mode of inheritance has also been indentified. Affected animals produce no antibodies after infection or immunization because of an absence of B lymphocytes and have no or nonfunctional T lymphocytes when present. This genetic disorder occurs when lymphocyte precursors fail to differentiate into mature T or B lymphocytes, which is likely because of the result of mutations within recombinase-activating genes or within genes encoding DNA-dependent protein kinase or when differentiated lymphocytes are incapable of completing signal transduction pathways because of defects in cell surface receptors for interleukins. Other examples of genetic alterations of the adaptive immune response that predisposes animals to increased susceptibility to infectious disease are discussed in the organ system chapters of this book. The pathogenicity (i.e., ability to cause disease) of a bacterium is regulated by its virulence determinates. Virulence determinates are molecules, often glycoproteins or glycolipids, derived from bacterial genes. Their expression establishes the processes used by bacteria to successfully colonize mucosae, infect cells, grow and replicate, and cause cell death. Virulence determinates are used to kill phagocytic cells such as neutrophils and macrophages, block phagocytosis, evade fusion with lysosomes and microbial killing, and enhance replication within phagocytes. Important actions (Fig. 4-13) of virulence determinates include the following: 1. Production of bacterial toxins that kill phagocytes. 2. Synthesis of bacterial proteins that prevent phagocytosis by blocking the interaction of opsonins with phagosomes. 3. Synthesis of a bacterial capsule that can block contact with the microbe and prevent phagocytosis. 4. Inhibition of fusion of the phagosome containing microbes with lysosomes. 5. Facilitate escape of the microbe into the cytoplasm before the microbe is killed in the phagolysosome. 6. Production of bacterial antioxidants (i.e., catalase) that block killing in phagolysosomes. Bacterial virulence determinates are molecules that influence interactions between bacteria and cells, including events such as adherence to cell membranes; mucosal colonization; cell entry via endocytosis or phagocytosis; growth and replication; local, regional, and systemic spread; and cell disruption, inflammation, injury, or death by acting as toxins (Fig. 4-14). In general, they are coded for by more than one microbial gene. Furthermore, they inhibit immune responses and allow the organism to hide from defense mechanisms and proliferate in harsh environments. Bacterial virulence is determined in part by the type and number of factors the bacterium expresses to successfully complete its life cycle in an animal. Other things that can indirectly influence the success of these virulence determinates include physical and environmental issues, such as weather, access to food and water, and management (shipping) or housing stressors (ventilation, humidity, or overcrowding). Some virulence determinates mediate adhesion, colonization, and invasion; others act indirectly to minimize the animal’s defense mechanisms by providing resistance to antibiotics, antiphagocytic properties, and weakened immune responses by acting as inhibitors. Adhesion, colonization, and invasion factors include bacterial membrane proteins, polysaccharide capsules, secretory proteins, cell wall and outer membrane components, and other miscellaneous molecules. Specific virulence determinates derived from membrane proteins assist the bacterium in adhering to, colonizing, and invading epithelia of barrier system portals of entry as in the alimentary, respiratory, integumentary, urinary, and reproductive systems. Because these epithelial cells are replaced continually (≈48 hour lifespan) and these systems have defensive mechanisms such as peristalsis, unidirectional mucociliary undulations, and urination, pathogenic bacteria must be able to adhere to, colonize (replicate), and/or invade into or between these epithelial cells to avoid being swept away. Attachment occurs when membrane proteins called adhesins (a broad term) bind to receptors on cell membranes. Attachment is a typical ligand-receptor interaction; a protein on the bacterium binds to a receptor on a host target cell. Some bacteria express adhesins, such as microbial surface cell recognition adhesion matrix molecules, that bind the bacterium to the surface of the cell. Other bacteria utilize extensions of their cell membranes called fimbriae or pili to bind to animal cells. Fimbriae and pili have adhesins, such as pilus-associated proteins, fimbrial antigens, or fimbrial adhesins, that bind to receptors on microvilli of the glycocalyx or in the mucus layer (Fig. 4-15). Fimbriae and pili may also act to inhibit phagocytosis. For example, uropathogenic and enterotoxic Escherichia coli, causes of urinary tract infections and diarrhea in animals, express fimbrial (type I, P, and S/F1C) and pilus (K99) adhesins, respectively. In the urinary tract, P-fimbriae is an important attachment adhesin and allows the bacterium to attach to the transitional epithelium and initiate the disease known as acute necrohemorrhagic urocystitis. Other virulence determinates, such as α-hemolysin and cytotoxic necrotizing factor type 1, cause necrosis and hemorrhage later. In the small intestine, K99 pilus adhesin allows E. coli to adhere to enterocytes and reduces their loss in number via peristalsis. When large numbers of E. coli are attached to the small intestine, they produce other virulence determinates, such as enterotoxin, that act directly on enterocytes, leading to diarrhea. Fig. 4-15 Fimbrial (pilus) and afimbrial adhesins. Ligand-receptor interactions are likely common to most bacterial diseases; however, in many veterinary diseases, specific bacterial ligands and their host cell receptors have not been identified. The attachment of sufficient numbers of bacteria at the appropriate portal of entry initiates an early stage of bacterial infection termed colonization. After colonization, another group of virulence determinates called invasins, or spreading factors, are produced by infectious organisms. These factors include hyaluronidase, collagenase, kinases, lecithinase, and phospholipase and act to break down barrier systems formed by mucosae (mucus layer) and skin, cell junctional complexes, and ECM molecules like collagen (Table 4-2). This process allows bacteria to spread rapidly into and through intercellular spaces and protect itself in safe areas isolated from unfavorable host environments or host-derived defensive molecules. As examples, Clostridium chauvoei, the bacterium that causes blackleg in cattle, produces sufficient lecithinases and phospholipases to punch holes in cell membranes and cause death of myocytes and endothelial cells. Listeria monocytogenes, the cause of listeriosis in the nervous system of cattle, produces invasins that induce endocytosis of the bacterium for colonization by acting on host cell actin filaments. Other proteins of bacteria, such as surface components and polysaccharide capsules, are virulence determinates that allow bacteria to avoid phagocytosis and evade recognition by cells of the innate and/or adaptive immune systems. They disrupt or block one or more steps used by neutrophils, monocytes, or macrophages in the phagocytic process such as initial contact, engulfment, phagosome formation, phagosome-lysosome fusion, and killing and digestion. As examples, Streptococcus pyogenes, a cause of bovine mastitis, utilizes M-protein and a hyaluronic acid capsule to inhibit phagocytosis and the same hyaluronic acid capsule to evade recognition of the immune system. TABLE 4-2 Biological Actions of Invasins or Spreading Factors Certain virulence determinates are toxins like exotoxins, lipoteichoic acid, and endotoxins (lipopolysaccharide [LPS]) that damage cells, and their ECMs, such as collagen, are expressed by Gram-negative and Gram-positive bacteria (Fig. 4-16). Functionally, these molecules act to kill cells via cytolysis cells or through activation of the inflammatory cascade, often initiated via the complement pathway. In certain diseases, these toxins are named (and grouped) according to their biologic activity such as leukotoxins and neurotoxins. Fig. 4-16 Morphology and molecules of Gram-positive and Gram-negative bacteria. Exotoxins and Lipoteichoic Acid: Exotoxins (usually from Gram-positive bacteria) are secreted from viable bacteria and are potent toxins (Fig. 4-17, A). Some act directly on cells to cause cytolysis; others act via the A-B toxin system and bind to cell membranes with a receptor (B subunit) and deliver a second toxic molecule (A subunit) into the cytoplasm. As examples, A-B toxin systems are used in botulism (Clostridium botulinum), tetanus (Clostridium tetani), and diseases caused by Corynebacterium spp. Another virulence determinate, lipoteichoic acid, is located in the cell wall of Gram-positive bacteria like Staphylococcus aureus. It behaves as a Gram-positive endotoxin because its actions mimic LPS. Lipoteichoic acid binds to endothelial cells, interacts with circulating antibodies, activates the complement cascade, and triggers the release of reactive oxygen and nitrogen species, acid hydrolases, highly cationic proteinases, bactericidal cationic peptides, growth factors, and cytotoxic cytokines from neutrophils and macrophages. Vacuolating toxin of Helicobacter pylori, E. coli hemolysin, and superantigens belonging to Streptococcus pyogenes and Staphylococcus aureus are surface-acting toxins. Surface-acting toxins bind to cell membranes and form pores through which cell death occurs. Staphylococcus aureus also has pore-forming cytotoxins called α-toxin. Fig. 4-17 Actions of bacterial toxins (virulence determinates) on the structure and function of target cells.
Mechanisms of Microbial Infections
Synopsis
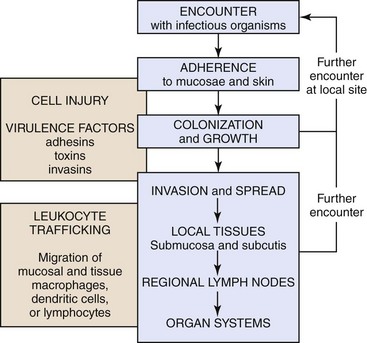
The chronologic sequence of events used by infectious microorganisms to colonize and invade mucosae and skin, spread to local tissues and regional and systemic organ systems, and cause disease. (Courtesy Dr. J. F. Zachary, College of Veterinary Medicine, University of Illinois.)
Portals of Entry
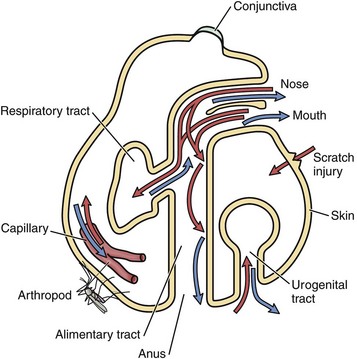
Infectious microorganisms commonly enter the body through ingestion (alimentary portal), inhalation (respiratory portal), cutaneous penetration (skin portal), and ascending infection (lower urinary and reproductive portals) and interact with epithelial cells, macrophages, dendritic cells, and lymphocytes of the mucosae or skin. (Modified from Goering R, Dockrell H, Roitt I, et al: Mims’ medical microbiology, ed 4, St. Louis, 2008, Mosby.)
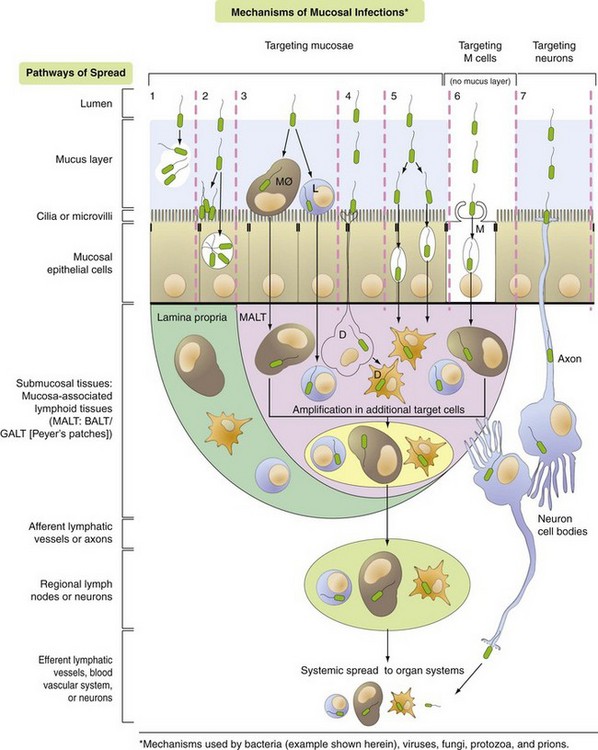
Pathway 1: Bacteria target the mucus layer.
Pathway 2: Bacteria target cilia or microvilli and/or mucosal epithelial cells.
Pathway 3: Bacteria target MALT via mucosal macrophages (MØ) and/or lymphocytes (L).
Pathway 4: Bacteria target MALT via dendritic cells (D).
Pathway 5: Bacteria target MALT via transcytosis or intercellular (junctional complexes) spread.
Pathway 6: Bacteria target MALT via M cells and transcytosis.
Pathway 7: Bacteria target nerve endings in mucosa and enter the brain via retrograde axonal transport. (Courtesy Dr. J. F. Zachary, College of Veterinary Medicine, University of Illinois.)
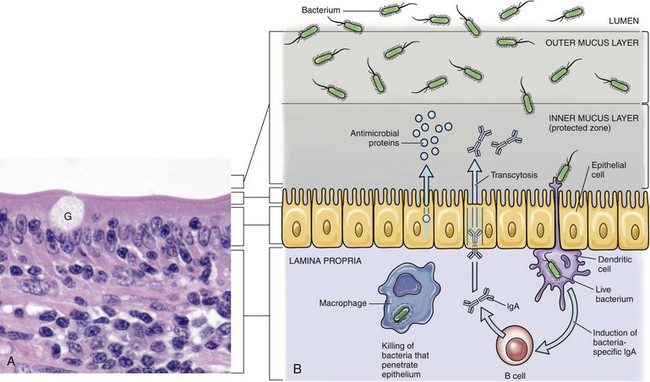
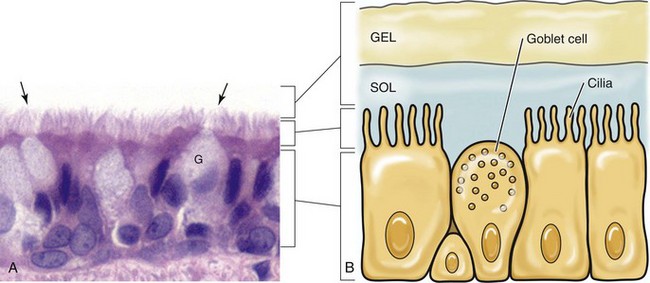
A, Mucosa of the intestine (shown here) and of the conductive respiratory airways are covered by a mucus layer (not visible in H&E sections) secreted by goblet cells (G). The mucus covers the microvilli or cilia of these systems. H&E stain. B, The mucus layer has an outer layer that traps microorganisms (infectious and noninfectious) and other particles and an inner layer in which the cilia beat and which contains antimicrobial substances that diffuse into the outer layer. Dendritic cells and mucosa-associated macrophages and lymphocytes play central roles in preventing infection of mucosa. (A courtesy Dr. J. F. Zachary, College of Veterinary Medicine, University of Illinois.)
Ingestion
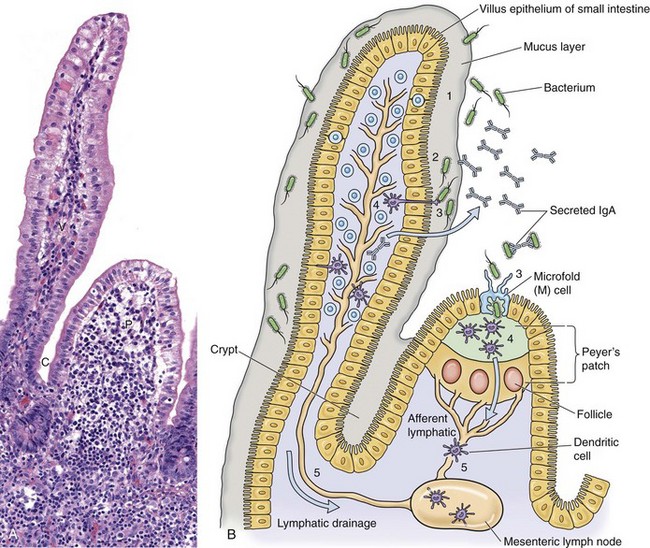
A, Mucosa that cover intestinal villi (V) and Peyer’s patches (P) and line crypts (C) form a barrier system that attempts to prevent the spread of infectious organisms into the underlying lamina propria. H&E stain. B, Schematic diagram of the responses of bacteria (or viruses) trapped in the mucus layer (1). Bacterial proteins (virulence determinates) act to allow them to penetrate the mucus layer and come into contact with the mucosal epithelium (2). IgA secreted by mature plasma cells in the lamina propria passes through mucosal epithelial cells into the lumen and can act as an “opsonizing” defense mechanism thus preventing infection. Bacteria then interact with mucosal epithelial cells, dendritic cells (D), or M cells (3). They then encounter lymphoid cells in the lamina propria or Peyer’s patches (4) and spread in lymphocytes or as free virus in lymph from this location via efferent lymphatic vessels to regional lymph nodes (5). Note the absence of a mucus layer over M cells and follicle-associated epithelium. Also see Fig. 4-7 for an example of barrier system: respiratory mucosae. (A courtesy Dr. J. F. Zachary, College of Veterinary Medicine, University of Illinois.)
Inhalation
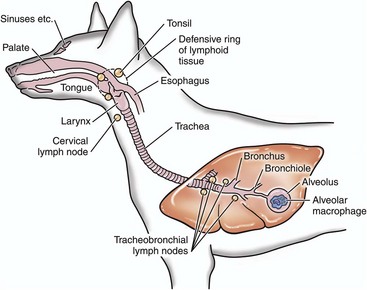
Infectious microorganisms inhaled through the nostrils are deposited on mucosa of the nasal turbinates, nasal pharynx, and/or the conductive system of the respiratory tract. The site of deposition depends on the physical properties of the agent such as size, shape, weight, and electrostatic charge. (Modified from Goering R, Dockrell H, Roitt I, et al: Mims’ medical microbiology, ed 4, St. Louis, 2008, Mosby.)
A, Cilia (arrows) of the bronchiole mucosal epithelial cells and the mucus layer (not visible) form the mucociliary apparatus of the conductive component of the respiratory system. The mucus layer is not visible because it has been removed during histologic processing of tissue. H&E stain. B, Diagram of the mucociliary apparatus. The mucus layer is biphasic and consists of a luminal viscoelastic or gel layer used to trap bacteria and a serous inner layer in which the cilia of ciliated mucosal epithelial cells beat unidirectionally to move bacteria upward in the airways to be swallowed or expectorated. G, Goblet cell. (Courtesy Dr. J. F. Zachary, College of Veterinary Medicine, University of Illinois.)
Cutaneous Penetration
Defense Mechanisms
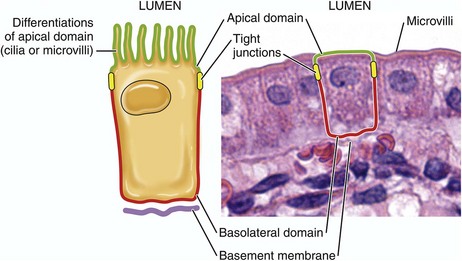
Infectious organisms use the apical or basolateral domains of mucosal epithelial cells to enter and exist from these cells. Apical or basolateral cell surface receptors may facilitate entry into the cell. (Courtesy Dr. J. F. Zachary, College of Veterinary Medicine, University of Illinois.)
Innate and Adaptive Immune Responses
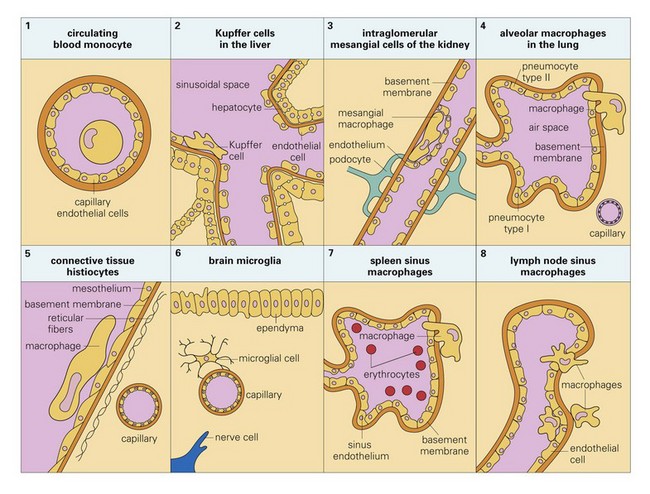
Monocyte-Macrophage System
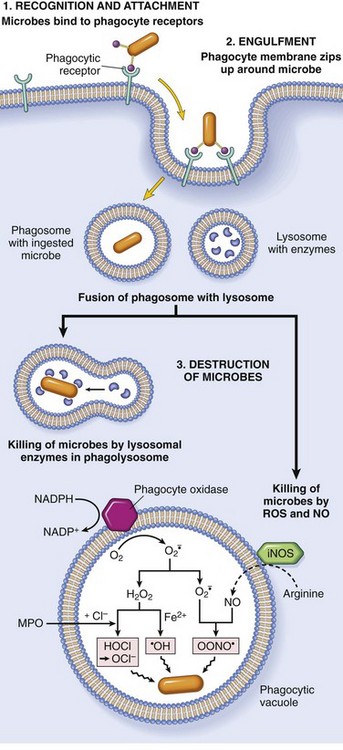
Phagocytosis of a particle (e.g., infectious microorganism) involves binding to receptors on the leukocyte membrane, engulfment, and fusion of lysosomes with phagocytic vacuoles. This process is followed by destruction of ingested particles within the phagolysosomes by lysosomal enzymes and by reactive oxygen and nitrogen species. The microbicidal products generated from superoxide are hypochlorite (HOCl·) and hydroxyl radical (·OH), and from nitric oxide (NO) it is peroxynitrite (OONO·). During phagocytosis, granule contents may be released into extracellular tissues (not shown). MPO, Myeloperoxidase; iNOS, inducible NO synthase. (From Kumar V, Abbas A, Fausto N, et al: Robbins & Cotran pathologic basis of disease, ed 8, Philadelphia, 2009, Saunders.)
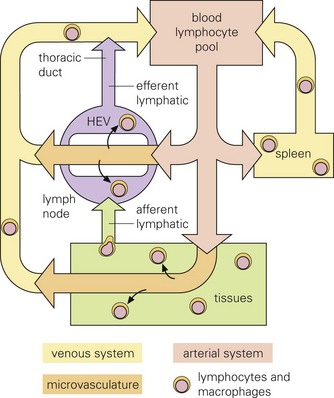
Infectious microorganisms often use macrophages, lymphocytes, and/or dendritic cells to spread themselves to other organ systems as these cells migrate through these systems as part of their normal immunologic surveillance activities. As an example, lymphocytes move through the circulation and enter lymph nodes via specialized endothelial cells of postcapillary venules (HEVs), leave through efferent lymphatic vessels and pass through other nodes, finally entering the thoracic duct, which empties into the circulation. Lymphocytes enter white pulp of the spleen, then pass into sinusoids of the red pulp and leave via the splenic vein. (From Goering R, Dockrell H, Roitt I, et al: Mims’ medical microbiology, ed 4, St. Louis, 2008, Mosby.)
Dendritic Cells
Genetic Resistance of Animals to Infectious Diseases
Disorders of Barrier Systems
Disorders of the Innate Immune Response
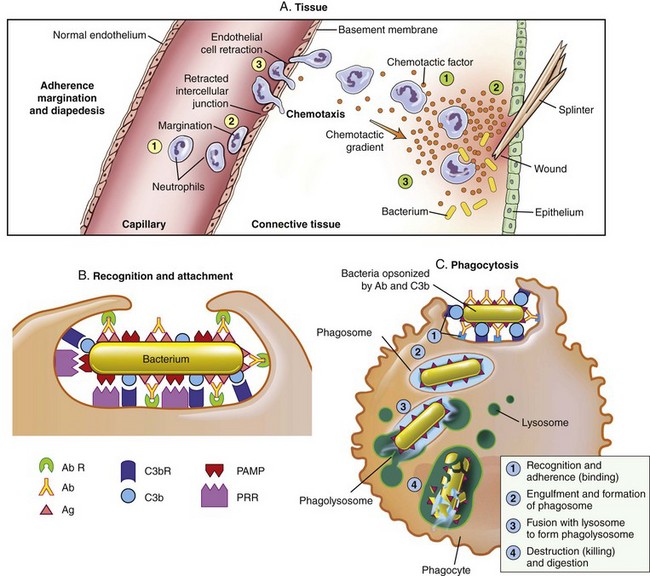
The process that results in phagocytosis is characterized by three interrelated steps: adherence and diapedesis, tissue invasion by chemotaxis, and phagocytosis. A, Adherence, margination, diapedesis, and chemotaxis. The primary phagocyte in the blood is the neutrophil, which usually moves freely within the vessel (1). At sites of inflammation, the neutrophil progressively develops increased adherence to the endothelium, leading to accumulation along the vessel wall (margination or pavementing) (2). At sites of endothelial cell retraction the neutrophil exits the blood by means of diapedesis (3). Chemotaxis: In the tissues, the neutrophil detects chemotactic factor gradients through surface receptors (1) and migrates towards higher concentrations of the factors (2). The high concentration of chemotactic factors at the site of inflammation immobilizes the neutrophil (3). B, Specific receptors for recognition and attachment. C, Phagocytosis. Opsonized microorganisms bind to the surface of a phagocyte through specific receptors (1). The microorganism is engulfed (ingested) into a phagocytic vacuole, or phagosome (2). Lysosomes fuse with the phagosome, resulting in the formation of a phagolysosome (3). During this process the microorganism is exposed to products of the lysosomes, including a variety of enzymes and products of the hexose-monophosphate-shunt (e.g., H2O2, O2−). The microorganism is killed and digested (4). Ab, Antibody; AbR, antibody receptor; C3b, complement component C3b; C3bR, complement C3b receptor; PAMP, pathogen-associated molecular pattern; PRR, pattern recognition receptor. (From McCance KL: Pathophysiology: the biologic basis for disease in adults and children, ed 6, St. Louis, 2010, Mosby.)
Disorders of the Adaptive Immune Response
Bacterial Diseases
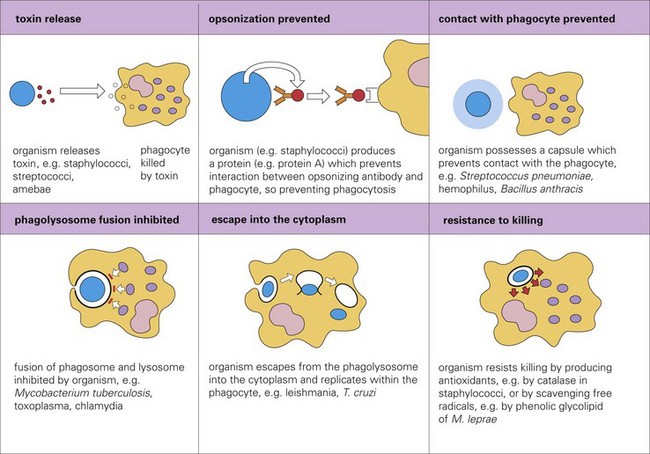
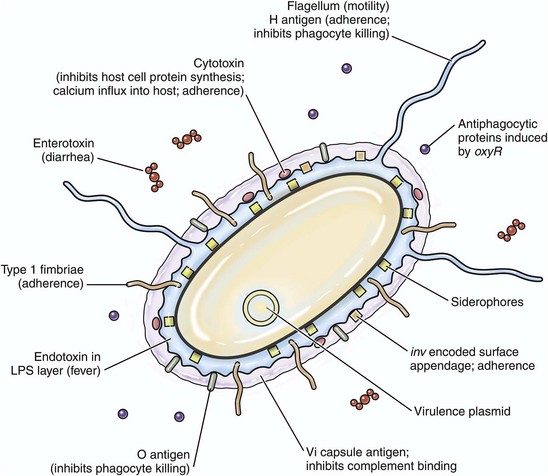
Virulence Determinates
Adhesion, Colonization, and Invasion
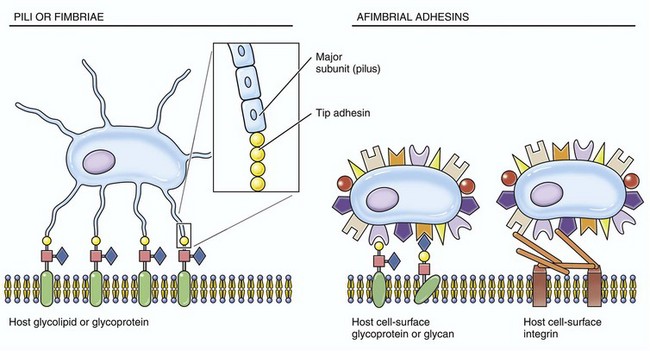
These structures are used by infectious microorganisms to attach and bind to protein receptors on membranes of target cells (especially mucosal epithelial cells) or to molecules of the mucus layer or vascularized extracellular matrix (connective) tissues.
Virulence Determinate
Action
Hyaluronidase
Depolymerizes hyaluronic acid in the extracellular matrix
Collagenase
Breaks down collagen fibers especially in muscle tissue
Neuraminidase
Degrades neuraminic acid (sialic acid) that serves to keep epithelial cells attached to basement membrane in the mucosa
Kinases
Digest fibrin and prevent clotting of the blood needed to wall-off bacteria
Lecithinase
Punch holes through or break down cell membranes
Phospholipase
Punch holes through or break down cell membranes
Toxins
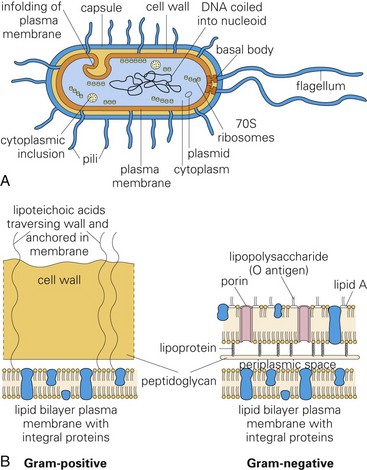
Molecules like exotoxins, lipoteichoic acid, and endotoxins (lipopolysaccharide [LPS]) that form the structure of bacterial cell walls are often toxic. They act as virulence determinates that damage cells and their extracellular matrices such as collagen. A, Morphology of a typical bacterium. B, Gram-positive bacteria have a thick layer of peptidoglycan (left). Gram-negative bacteria have a thin peptidoglycan layer and an outer membrane of LPS (right). (A and B from Goering R, Dockrell H, Roitt I, et al: Mims’ medical microbiology, ed 4, St. Louis, 2008, Mosby.)
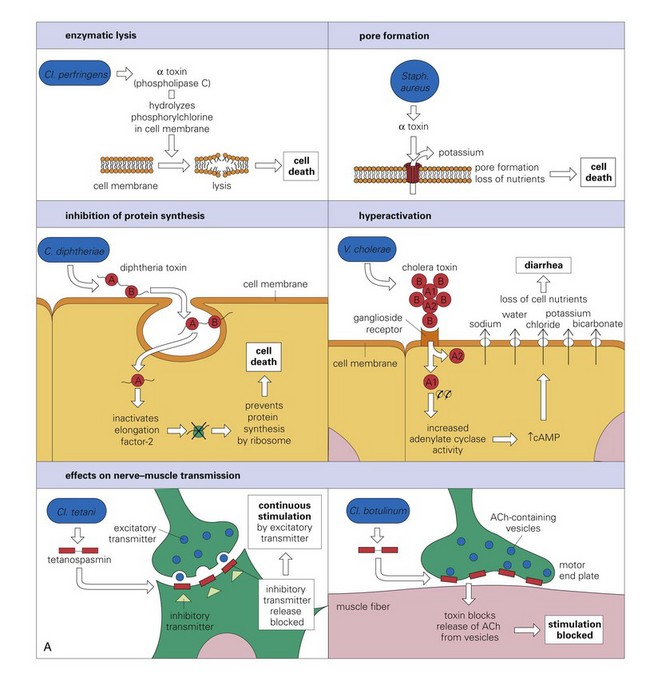
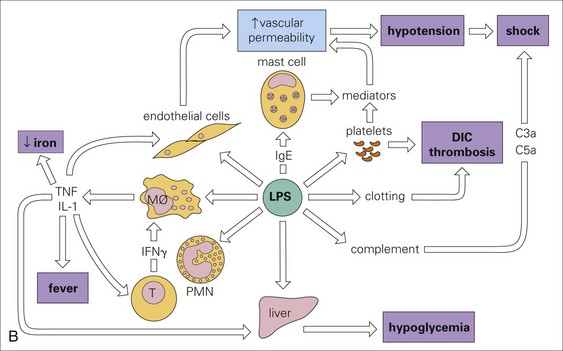
A, The mode of action of some exotoxins. Bacterial toxins act in a variety of ways. Often the toxin is a two-chain molecule, one chain being concerned with entry into cells while the other has inhibitory activity against some vital function. ACh, Acetylcholine; cAMP, cyclic adenosine monophosphate; C, Corynebacterium; Cl, Clostridium; Staph, Staphylococcus; V, Vibrio. B, The activities of bacterial endotoxin. Lipopolysaccharide (LPS) activates almost every immune mechanism, as well as the clotting pathway, and as a result, LPS is one of the most powerful immune stimuli known. DIC, Disseminated intravascular coagulation; IFN, interferon; IL, interleukin; M, macrophage; PMN, polymorphonuclear leukocyte; TNF, tumor necrosis factor. (A and B from Goering R, Dockrell H, Roitt I, et al: Mims’ medical microbiology, ed 4, St. Louis, 2008, Mosby.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree