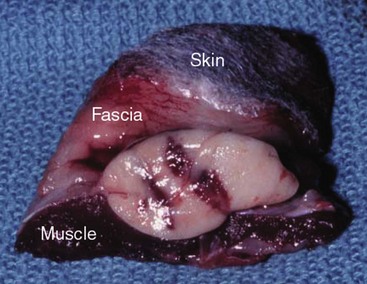
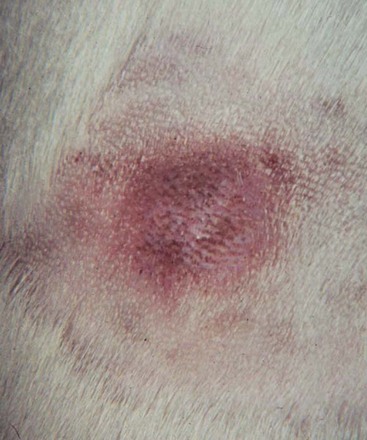
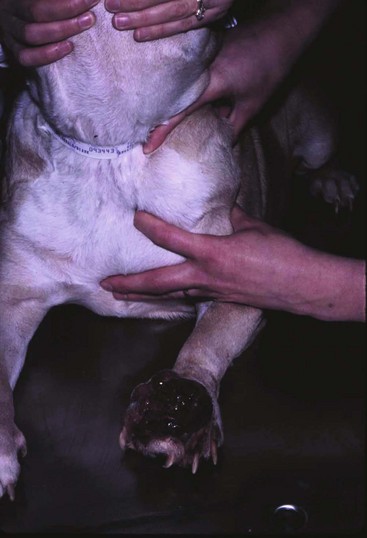
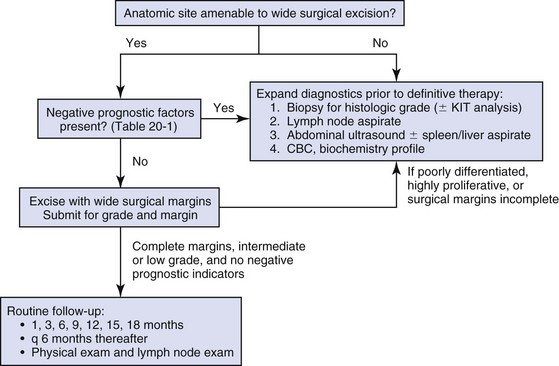
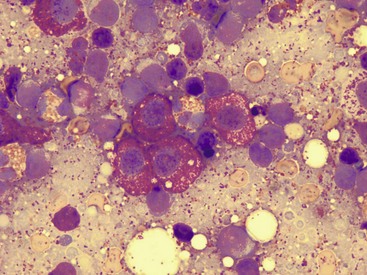
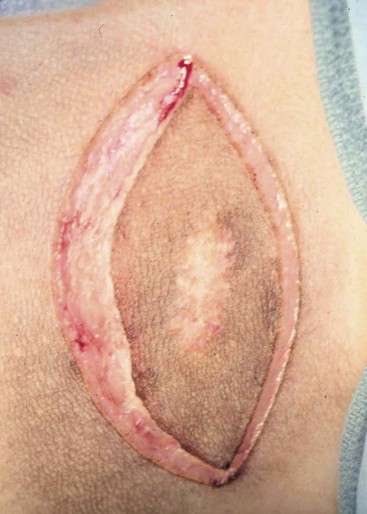
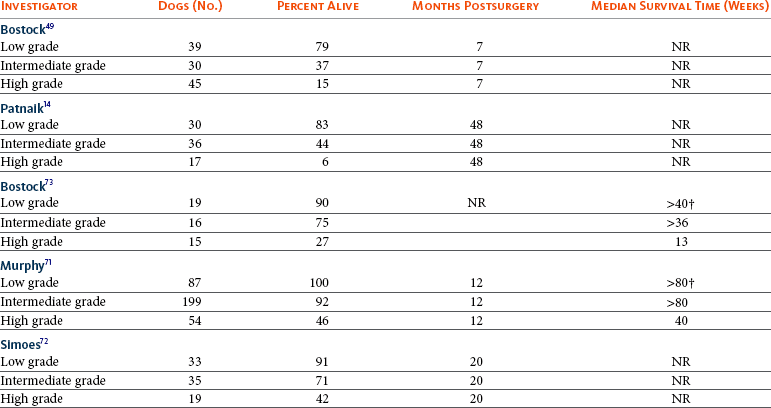
20 The neoplastic proliferation of mast cells referred to as a mast cell tumor (MCT; histiocytic mastocytoma, mast cell sarcoma) represents the most commonly encountered cutaneous tumor in the dog and the second most common cutaneous tumor in the cat.1–5 Systemic forms of the disease are often referred to as mastocytosis. Canine and feline forms of the disease will be considered separately in this chapter because many differences exist with regard to histologic type, biologic behavior, therapy, and prognosis. Mast cell precursors leave the bone marrow and migrate to various tissues throughout the body where they undergo differentiation into mature mast cells with their characteristic cytoplasmic granules, which stain metachromatically with Giemsa and toluidine blue.6 These granules contain a number of bioactive substances, including heparin, histamine, preformed tumor necrosis factor-α (TNF-α), and several proteases.6 The nature and composition of mast cell granules are highly influenced by the microenvironment in which the mast cells mature. For example, in dogs, mast cells in the gastrointestinal (GI) tract express primarily chymase, whereas mast cells in the skin express both chymase and tryptase.7 When stimulated, mast cells can rapidly produce a variety of proteases (chymase, tryptase), cytokines (TNF-α, interleukin-6 [IL-6]), chemokines (CCL2, CXCL1), growth factors (vascular endothelial growth factor [VEGF], basic fibroblast growth factor [bFGF]), and lipid mediators (prostaglandin D2 [PGD2], leukotriene C4 [LTC4]).6 Through this process, mast cells participate in several biologic activities, including wound healing, induction of innate immune responses, antiparasite activity, and modulation of reaction to insect and spider venoms.6,8 Normal canine mast cells can be generated from bone marrow–derived hematopoietic precursors.9,10 These cells, known as canine bone marrow–derived mast cells (cBMMCs), have been used to characterize the functional properties of mast cells in this species. As with normal human mast cells, their differentiation requires the presence of the growth factor stem cell factor (SCF) and can be influenced by the presence of other cytokines.9,10 When stimulated, these cells rapidly release histamine, monocyte chemotactic protein-1 (MCP-1), TNF-α, and tryptase, and they produce several additional cytokines and chemokines, including IL-3, IL-13, granulocyte-macrophage colony-stimulating factor (GM-CSF), and macrophage inflammatory protein 1α (MIP1α).9,10 Interestingly, the cBMMCs are extremely sensitive to chemical degranulation, which may help to explain why dogs exhibit such a high degree of hypersensitivity to several chemical agents, including polysorbate 80, Cremophor EL, and doxorubicin.9 The function of cBMMCs can be modulated by cytokines, steroids, and nonsteroidal antiinflammatory drugs (NSAIDs).9–11 MCTs represent the most common cutaneous tumor in the dog, accounting for between 16% and 21% of all cutaneous tumors.1,3,5,12 Although MCTs are primarily a disease of older dogs (mean age approximately 8 to 9 years), they have also been reported in younger dogs and there is no apparent gender predilection.1,3,5,13 Most tumors occur in mixed breeds; however, several breeds are at an increased risk for MCTs, including dogs of bulldog descent (Boxers, Boston terriers, English bulldogs, pugs), Labrador and golden retrievers, cocker spaniels, schnauzers, Staffordshire terriers, beagles, Rhodesian ridgebacks, Weimaraners, and Shar-Peis.1,5,14–16 The increased incidence of MCTs in certain breeds suggests the possibility of an underlying genetic cause,15 and studies are ongoing to identify these putative genetic risk factors. Interestingly, although dogs of bulldog ancestry are at higher risk for MCT development, it is generally accepted that MCTs in these dogs are more likely to behave in a benign fashion.1 Additionally, it was recently demonstrated that pugs may develop multiple MCTs that behave in a benign fashion.17 In contrast, anecdotal evidence suggests that Shar-Peis develop MCTs that may be more biologically aggressive. Spontaneously regressing MCTs in young animals have been described in cats, pigs, horses, and humans. Multiple cutaneous MCTs that regressed within 27 weeks were reported in a 3-week-old Jack Russell terrier.18 This syndrome of spontaneous regression in young animals may indicate a hyperplastic or dysplastic syndrome rather than a true neoplastic lesion. The etiology of MCTs in the dog is for the most part unknown. Historically, MCTs have been associated with chronic inflammation or the application of skin irritants; however, the epidemiology of disease in dogs does not support the role of a topical carcinogen.19–21 Unequivocal evidence is lacking for a viral etiology, although MCTs have been transplanted to very young or immunocompromised laboratory dogs using tumor cell tissues and rarely by cell-free extracts.22–24 No C-type or other identifiable virus particles have been observed, and no epidemiologic evidence exists to suggest horizontal transmission. Chromosomal fragile site expression, a phenomenon thought to genetically predispose humans to develop certain tumors, was shown to be increased in Boxers with MCTs.25 However, the control population for this study was young, non–tumor-bearing Boxers, and the increased expression of chromosomal fragile sites may be due to this age difference. The genetic changes that predispose dogs to MCTs are incompletely understood. Alterations in the p53 tumor suppressor pathway have been identified in some canine MCTs,26–28 but p53 sequencing in a limited number of cases has revealed no mutations.29 Perturbations in expression of the proteins p21 and p27, which are cyclin-dependent kinase inhibitors (CDKIs) that contribute to regulation of the cell cycle, have been identified in many canine MCTs.30 Cytosolic receptors for estrogen and progesterone have also been detected in canine MCTs.31 Their role in the etiopathogenesis of MCT is poorly understood, but evidence exists that estrogen and progesterone may influence mast cell function.32 One European study reported that female dogs with MCTs had a more favorable prognosis with chemotherapy.33 Although the majority of studies performed in the United States have failed to detect such an association, the relatively higher frequency of intact females present in the European population may have allowed the effect of sex hormones to have a greater statistical impact on biologic behavior. Perhaps the best-described molecular abnormality in canine MCT involves the receptor tyrosine kinase (RTK), KIT. KIT is expressed normally on a variety of cells, including hematopoietic stem cells, melanocytes, and mast cells, among others.34–36 The ligand for KIT, SCF, induces KIT dimerization, subsequent phosphorylation, and generation of intracellular signaling that promotes the proliferation, differentiation, and maturation of normal mast cells.34–36 SCF is essential for the differentiation of mature mast cells from CD34+ hematopoietic stem cells in vitro, and inhibition of KIT signaling induces apoptosis of cBMMCs.10,35,36 KIT expression has been demonstrated on canine MCTs, and aberrant cytoplasmic localization of KIT in MCTs may be associated with dysregulated KIT function.37–40 A significant proportion of canine MCTs possess mutations in the c-kit gene involving either the juxtamembrane domain (exons 11 and 12) or extracellular domain (exons 8 and 9).41–45 These mutations result in SCF-independent activation of KIT and subsequent unregulated KIT signal transduction.43,44 In dogs, activating c-kit mutations appear to be present in 25% to 30% of intermediate-grade and high-grade MCTs, and evidence suggests that they are linked to increased risk of local recurrence, metastasis, and a worse prognosis.41,44,46–48 The reason for the high rate of c-kit mutations in dog MCTs is unknown; however, it is clear that these mutations are not germline in nature. The vast majority of MCTs in dogs occur in the dermis and subcutaneous tissue,5,49 and most are solitary in nature, although 11% to 14% of dogs present with multiple lesions.50–52 Approximately 50% of cutaneous MCTs occur on the trunk and perineal region, 40% on the limbs, and 10% on the head and neck.20,53,54 MCTs have also been reported to occur on other sites, including the conjunctiva, salivary gland, nasopharynx, larynx, oral cavity, ureter, and spine.55–59 A visceral form of MCT, often referred to as disseminated or systemic mastocytosis, has also been documented, although it is usually preceded by an aggressive primary lesion.60–64 Infiltration of abdominal lymph nodes, spleen, liver, and bone marrow is commonly observed in the setting of visceral disease, and pleural/peritoneal effusions containing neoplastic mast cells have been documented. A case series of dogs with primary GI MCTs was recently reported in which most dogs presented with vomiting, diarrhea, and melena. Only 40% of the dogs were alive at 30 days after first admission, and fewer than 10% were alive at 6 months.28 It is important to note that cutaneous MCTs have an extremely varied range of clinical appearances, and they are sometimes inadvertently mistaken for nonneoplastic lesions. Well-differentiated MCTs tend to be solitary, small, slow-growing tumors that may have been present for several months. They are not typically ulcerated, but overlying hair may be lost. Undifferentiated MCTs tend to be rapidly growing, ulcerated lesions that cause considerable irritation and attain a large size. Surrounding tissues may become inflamed and edematous. Small satellite nodules may develop in surrounding tissues. Tumors of intermediate differentiation fill the spectrum between these two extremes. A subcutaneous form of MCT that is soft and fleshy on palpation is often misdiagnosed clinically as a lipoma (Figure 20-1). The history and clinical signs of dogs with MCTs may be complicated by signs attributable to the release of histamine, heparin, and other vasoactive amines from mast cell granules. Occasionally, mechanical manipulation during examination of the tumor results in degranulation and subsequent erythema and wheal formation in surrounding tissues. This phenomenon has been referred to as Darier’s sign (Figure 20-2)51 and can also occur spontaneously; dog owners may describe the tumor as increasing and decreasing in size. GI ulceration has been documented in 35% to 83% of dogs with MCTs that underwent necropsy.65,66 Histamine released from MCT granules is thought to act on parietal cells via H2 receptors, resulting in increased hydrochloric acid secretion. Plasma histamine concentrations have been shown to be high in dogs with MCTs, and there is preliminary information that monitoring of plasma histamine concentrations may be useful in assessing disease progression.67 These dogs also have decreased concentrations of plasma gastrin, which is normally released by antral G-cells in response to increased gastric hydrochloric acid concentrations, acting as a negative feedback loop. Dogs with substantial MCT burden (i.e., large tumors, metastatic disease, systemic disease) are much more likely to present with clinical signs related to the release of mast cell mediators. These may include vomiting, diarrhea, fever, peripheral edema, and, rarely, collapse. Perioperative degranulation of MCTs and subsequent release of histamine and other less-characterized vasoactive substances may also result in potentially life-threatening hypotensive events during surgery. It is thought that prostaglandins in the D series secreted by tumor cells may mediate the hypotensive effects observed in humans with mast cell diseases.68,69 Coagulation abnormalities, also reported in dogs with MCTs, are likely due to heparin release from mast cell granules.63,70 Although clinical evidence of hemorrhage is not typically associated with this phenomenon, localized hemorrhage at the time of surgery as the result of degranulation following tumor manipulation can be a serious complication, even in the presence of normal presurgical coagulation parameters. A discussion of prognostic factors associated with canine MCTs will precede sections on diagnosis and treatment because steps followed in those sections are predicated on the presence or absence of these prognostic factors. Table 20-1 lists factors known to be predictive of biologic behavior and clinical outcome in dogs with MCTs. It is important to note that no one factor is entirely predictive of biologic behavior; thus all prognostic indicators should be taken into consideration when evaluating a patient. Table 20-1 Prognostic Factors for Mast Cell Tumors in Dogs Histologic grade is considered the most consistent and reliable prognostic factor available for dogs with MCTs, although it will not predict the behavior of every tumor.14,49,71,72 Several investigators have applied histologic grading systems to canine MCTs based on degree of differentiation (Table 20-2). The number grades used in these studies are at odds. Therefore, for the sake of clarity, the three differentiation groups should be simply referred to as undifferentiated (high) grade, intermediate grade, and well-differentiated (low) grade. Table 20-3 lists the relative distribution of MCT grades encountered in larger series. Survival following surgical excision based on grade is presented in Table 20-4. The vast majority of dogs with well-differentiated tumors (80% to 90%) and approximately 75% of dogs with intermediate-grade tumors experience long-term survival following complete surgical excision.50,71,74–77 Metastatic rates for undifferentiated tumors range from 55% to 96%, and most dogs with these tumors die of their disease within 1 year.49,78 The majority disseminate first to local lymph nodes, then to the spleen and liver. Other visceral organs may be involved; however, lung involvement is infrequent. Neoplastic mast cells may be observed in the bone marrow and peripheral blood in cases of widespread systemic dissemination.63 Table 20-4 Survival Times of Dogs with Surgically Treated Mast Cell Tumors According to Histologic Grade* *Unclear in these studies if death due to metastasis or local recurrence. †Medians not reached at the time of last follow-up (i.e., >50% alive). The current histopathologic grading system does not detect a small percentage (15% to 30%) of those well-differentiated or intermediately differentiated MCTs that result in the death of affected dogs; this is complicated by the fact that there is disagreement in tumor grading among pathologists. In one study, there was significant variation among pathologists in grading a specific set of MCTs (p < 0.001), although this was found to be less so if all pathologists strictly employed the system described by Patnaik.14,79,80 Recently, an attempt was made to develop a new grading system that would separate tumors into high or low grade based on one of four features identified on histopathologic evaluation.81 In this setting, tumors would be classified as high grade if they possessed (1) at least seven mitotic figures/10 HPF, (2) at least three multinucleated cells/10 HPF, (3) at least three bizarre nuclei/10 HPF, or (4) karyomegaly. In a series of 95 dogs evaluated by both the Patnaik system and this alternative system, the alternative system was somewhat better at predicting which dogs would be more likely to die of disease81; however, further validation of this new two-tiered system will be necessary to determine whether it is truly better than the more commonly used Patnaik three-tiered system for predicting the biologic behavior of MCTs. Several markers of proliferation have been evaluated to assist in determining whether an MCT is likely to behave in a more aggressive manner.47,72,82–90 Ki67 is a protein found in the nucleus, the levels of which appear to correlate with cell proliferation. In one study, the mean number of Ki67-positive nuclei was significantly higher for dogs that died of MCTs than for those that survived.83 In another study, the Ki67 score was used to divide intermediate-grade MCTs into two groups with markedly different expected survival times.84 Silver colloid staining of paraffin-embedded sections can be used to determine the relative presence of argyrophilic nucleolar organizer regions (AgNORs), another surrogate marker of proliferation. These have been correlated with histologic grade and postsurgical outcome.72,73 In a study of 50 dogs with cutaneous MCTs, the AgNOR frequency was as predictive or more predictive of biologic behavior than histologic grade.73 Finally, proliferating cell nuclear antigen (PCNA), another indicator of cell proliferation, has been used to determine the biologic behavior of MCTs, although this is probably not as reliable as the other markers.72,73,91 The previously discussed markers of proliferation all require the use of special stains. In contrast, the mitotic index (MI), or number of mitoses/10 HPF in hematoxylin and eosin (H&E) sections, has been used to assess the biologic behavior of canine MCTs.92 In one study, those dogs with tumors possessing an MI of 5 or lower had a median survival time (MST) of 80 months, compared to 3 months for those possessing an MI of more than 5, suggesting that the MI is a strong predictor of overall survival for dogs with MCTs. Additional studies have also found a role for MI in MCT prognosis.81,90,92–95 Other cellular assessments have been employed to evaluate the biologic behavior of MCT. A study of DNA ploidy determined by flow cytometric analysis suggested a trend toward shorter survival and higher clinical stage of disease in aneuploid tumors compared to diploid tumors.96 Recent studies have found a correlation between intratumor microvessel density and invasiveness, mitotic rate, and prognosis82,97 and a correlation between nuclear characteristics (assessed by computerized morphometry) and outcome and grade.98,99 The potential role of KIT dysregulation in MCT prognosis was investigated by assessing KIT immunohistochemical staining patterns on histopathologic specimens.100 Three distinct patterns were identified: membrane, focal/stippled, and diffuse cytoplasmic staining. Although there was some evidence that dogs with diffuse cytoplasmic KIT staining patterns did not live as long as those with other patterns, no group reached an MST and most dogs in each of the KIT staining groups evaluated experienced extremely long survival times postsurgery.100 In contrast, the presence of c–kit–activating mutations has been associated with a higher rate of local recurrence, metastasis, and death from disease, suggesting that KIT dysregulation confers a more aggressive phenotype to MCTs.41,46,47 Finally, investigators have attempted to correlate histologic grading of MCTs with a combined Ki67/PCNA/AgNOR/KIT immunohistochemical scoring.101 No significant correlation was found for KIT staining and MCT grade, but high Ki67/PCNA/AgNOR scores all did correlate positively with tumor grade (i.e., higher scores for higher grade). This suggests that proliferation indices increase with increasing grade and are ultimately reflected in the eventual biologic behavior of the tumor. Tumor location has been investigated as a potential prognostic indicator.50,102–108 Tumors in the preputial/inguinal area, subungual (nailbed) region (Figure 20-3), and other mucocutaneous sites, including the oral cavity and perineum, have been associated historically with aggressive behavior. Two reports did not show a negative prognosis for tumors occurring in the inguinal and perineal region; however, when preputial and scrotal regions were specifically evaluated, they were indeed associated with a poorer prognosis.106,107 Approximately 50% to 60% of dogs with MCTs located in the muzzle present with regional lymph node metastasis.108,109 Interestingly, this does not necessarily indicate a worse long-term prognosis because the MST for dogs with metastatic disease was 14 months.108 MCTs that originate in the viscera (GI tract, liver, spleen) or bone marrow carry a grave prognosis.62,63 Recent data indicate that MCTs arising in the subcutaneous tissues have a favorable prognosis, with extended survival time and low rates of recurrence and metastasis. In one study of 306 dogs with subcutaneous MCTs, metastasis occurred in 4% of dogs and 8% experienced local recurrence.95 The estimated 2- and 5-year survival probabilities were 92% and 86%, respectively. Decreased survival was linked to MI higher than 4, infiltrative growth pattern, and presence of multinucleation. Finally, conjunctival MCTs were found to have a good prognosis, with 15/32 dogs disease free at a mean of 21.4 months postsurgery; no dogs in this study died of MCT-related disease.110 Clinical stage, represented in Table 20-5, is also predictive of outcome.33,49,96,105,111 There is controversy regarding the effect of multiple MCTs on prognosis, and as such, this part of the staging scheme may not accurately correlate with outcome. Several studies indicate that there is no difference in outcome between patients with a single cutaneous MCT and those with multiple MCTs,50,112–114 whereas others have suggested an inferior outcome in dogs with multiple tumors.94,115 It is uncertain at this time whether this phenomenon represents an atypical form of metastasis or multiple, unrelated tumors arising independently, although one study demonstrated a clonal origin for two distant cutaneous tumors arising over years.116 The effect of lymph node metastasis on prognosis is also somewhat controversial. In two studies, the presence of mast cells in the regional lymph node was a negative prognostic factor for survival and disease-free interval (DFI)113,117; however, an additional study revealed that dogs with intermediately differentiated MCTs and lymph node metastasis treated with radiation therapy (RT) postsurgery achieved long-term survival.118 Other studies have shown that dogs with intermediately differentiated MCT with lymph node metastasis may have a good prognosis if the affected lymph node is removed and adjuvant chemotherapy is administered.113,114,119 For poorly differentiated tumors, the presence of metastatic disease resulted in an MST of 194 days compared to 503 days for dogs with no metastasis.78 For these dogs, treatment of the lymph node improved survival time (240 days) compared to those dogs whose lymph nodes were not treated (42 days).78 As with all cases, clinical judgment regarding lymph node metastasis is probably important. A dog with an effaced enlarged lymph node will be more likely to fail therapy when compared to a dog with a lymph node that is not clinically enlarged but has cytologic evidence of metastasis. Several miscellaneous factors have been linked to prognosis in dogs with MCTs. Certain breeds of dogs such as Boxers, pugs, and dogs of bulldog descent appear to develop MCTs that often behave in a more benign fashion.1,17,49 Recent rapid growth has been associated with a worse outcome. For example, in one study, 83% of dogs with tumors present for longer than 28 weeks prior to surgery survived for at least 30 weeks, compared to only 25% of dogs with tumors present for less than 28 weeks.49 Systemic signs of anorexia, vomiting, melena, widespread erythema, and edema associated with vasoactive substances from mast cell degranulation are more commonly associated with visceral forms of MCT and carry a more guarded prognosis.50,63,64 In 16 cases of visceral MCTs, an MST of 90 days was reported and all dogs with follow-up died of their disease.103 Local tumor ulceration, erythema, or pruritus has been associated with a worse prognosis in some studies.50,113 Finally, recurrence of MCT following surgical excision has also been associated with a more guarded prognosis.113 Thus appropriate aggressive therapy at the time of first presentation, rather than at the time of recurrence, may improve the long-term prognosis in patients with MCTs. MCTs are initially diagnosed on the basis of fine-needle aspiration (FNA) cytology. The Romanowsky or rapid hematologic-type stains used in most practices will suffice. Mast cells appear as small to medium-sized round cells with abundant, small, uniform cytoplasmic granules that stain purplish red (metachromatic; see Figures 7-2 and 7-5).1,120 A small percentage of MCTs have granules that do not stain readily, giving them an epithelial or macrophage-like appearance that has often been referred to as a “fried-egg” appearance (see Figure 7-2). In these cases, a Wright-Giemsa or toluidine blue stain will often reveal granules; however, histologic assessment may ultimately be necessary. Highly anaplastic, agranular MCTs can sometimes be challenging to definitively diagnose by routine light microscopy. Immunohistochemical techniques have been applied in an attempt to differentiate these from other anaplastic round cell tumors. MCTs are vimentin positive, and the majority are tryptase and CD117 (KIT) positive.37,121–123 Other markers that could potentially be useful include chymase, MCP-1, and IL-8.9,10 Historically, complete staging has included a minimum database (complete blood count [CBC], serum biochemistry profile), a buffy coat smear to document peripheral mastocytosis, cytologic assessment of regional lymph nodes, abdominal ultrasound (US) with cytologic assessment of spleen or liver if warranted, thoracic radiographs, and bone marrow aspiration cytology. It is now likely that an extensive work-up is not necessary for dogs with MCTs that do not exhibit the previously discussed negative prognostic factors. Figure 20-4 illustrates the diagnostic steps and the order in which they are pursued in the authors’ practice. If the MCT is in a location amenable to wide surgical excision and no negative prognostic indicators are present (see Table 20-1), no further tests other than the minimum database and FNA of the regional lymph node (if possible) are performed prior to wide surgical excision. FNA cytology is not sufficient to grade MCT; thus histologic assessment following excision is strongly recommended to provide guidance regarding necessary further diagnostics and therapeutics. If the tumor presents at a site that is not amenable to wide surgical excision (e.g., distal extremity) or if negative prognostic factors exist in the history or physical examination, ancillary diagnostics to further stage the disease are recommended prior to definitive therapy. An incisional/needle biopsy may be performed at this point for determination of histologic grade. The minimum staging that is advisable in those cases requiring presurgical staging consists of a minimum database, FNA cytology of the regional lymph node (even if normal size), and abdominal US. With respect to cytologic evaluation of lymph nodes, definitive criteria for metastatic disease can be challenging if mast cells are present in low numbers because mast cells are normally found in lymph nodes and their numbers can be increased in the presence of infection and ulceration, which are sometimes observed in MCTs. For example, in 56 healthy beagle dogs, approximately 24% of lymph node aspirates contained mast cells (range of 1 to 16 mast cells/slide, mean of 6.4 cells/slide).124 Therefore an occasional solitary mast cell is not indicative of metastasis; rather, clustering and aggregates are more worrisome (Figure 20-5).111 Surgical removal of a cytologically suspicious lymph node for histologic assessment may be necessary to accurately determine whether mast cells present in the lymph node truly represent metastatic disease. Abdominal US is now considered an important diagnostic test for the evaluation of dogs with potentially aggressive MCTs. Although FNA cytology of structurally normal livers or spleens is generally unrewarding,125,126 the presence of negative prognostic indicators (e.g., metastatic lymph node, clinical signs) is sufficient justification to perform cytologic evaluation of these organs even if they appear normal on US.127 Thoracic radiographs rarely demonstrate metastasis; however, it is reasonable to procure them prior to an expensive or invasive procedure to rule out occult cardiopulmonary disease that could complicate anesthesia or unrelated disease processes (e.g., primary lung tumor). Occasionally, thoracic lymphadenopathy may be observed as a result of MCT metastasis. Knowledge of the extent of MCT margins prior to surgery, usually accomplished by digital palpation and occasionally local radiographs, can be enhanced with the use of diagnostic US or computed tomography (CT). Dogs bearing cutaneous MCTs or soft tissue sarcomas had the extent of local tumor margins upgraded in 19% and 65% of cases when imaged by US and CT, respectively.128 Such information allows more appropriate planning of definitive surgery or RT. The cost effectiveness of such a study depends on the location of the tumor and whether wide excision is technically simple or difficult. With respect to evaluation of buffy coat smears for evidence of systemic mast cell disease, peripheral mastocytosis (1 to 90 mast cells/µL) is reported in dogs with acute inflammatory disease (in particular, parvoviral infections), inflammatory skin disease, regenerative anemias, neoplasia other than MCTs, and trauma.129–131 One study revealed that peripheral mastocytosis is actually more likely to occur and may be more dramatic in dogs with diseases other than MCTs.130 Therefore this test is no longer routinely performed in the staging of MCT patients. In a report evaluating 157 dogs with MCTs, the incidence of bone marrow infiltration at initial staging was only 2.8%.132 Although the presence of bone marrow involvement is indicative of systemic mast cell disease, it is usually easier to find evidence of systemic involvement in other organs (liver, spleen).63 This is in contrast to dogs that present with visceral MCTs, in which 37% of buffy coat smears are positive for mast cells and 56% of bone marrow aspirates reveal mast cell dissemination103; however, these constitute a small minority of all MCT cases. Therefore, with the exception of the extremely rare case of primary mastocytic leukemia,133,134 involvement of marrow or peripheral blood in the absence of disease in regional lymph node or abdominal organs is unlikely.132 Treatment decisions are predicated on the presence or absence of negative prognostic factors and on the clinical stage of disease. In tumors localized to the skin in areas amenable to wide excision, surgery is the treatment of choice. Historically, surgical excision to include a 3-cm margin of surrounding normal tissue has been recommended for MCT. More recently, evidence exists that 1- to 2-cm lateral margins may be sufficient for complete excision of many MCTs, particularly those that are lower grade and small.135,136 Indeed, in 100 dogs with 115 resectable MCTs (primarily intermediate and low grade), no local recurrence or metastasis was noted for greater than 2 years following excision with lateral histologic margins of 10 mm or larger and deep histologic margins of 4 mm or larger.76 It should be noted that these microscopic, formalin-fixed margin parameters may not accurately reflect margin size at surgery: tissue shrinkage (up to 30% for cutaneous tissues) can occur subsequent to formalin fixation.137–139 Considering that the majority of naïve dermal MCTs encountered in practice are intermediate- or low-grade tumors, it can be said that most MCTs can be cured with surgery alone, provided the site is amenable to adequate surgical resection. The quality of the deep margin is as important as that of the lateral margins, and it is recommended that one uninvolved fascial plane deep to the tumor be removed in continuity with the tumor. If necessary, muscle layers may also be removed deep to the tumor. All surgical margins should be evaluated histologically for completeness of excision. It is recommended that tumors in areas not amenable to wide surgical margins, such as distal extremities, be evaluated by biopsy to determine histologic grade prior to definitive therapy. If a distal extremity MCT is low or intermediate grade and complete excision is not achievable, four primary therapy options exist. The most aggressive option is amputation; however, although wide margins are guaranteed, it results in the least functional outcome and is generally not recommended given the availability of other effective therapies. The second option is external-beam RT alone, which produces varying control rates in the literature when used as a primary therapy; doses between 40 and 50 Gy result in 1-year control rates of approximately 50%.105,140–145 The third and, in the authors’ opinion, the ideal option for low- or intermediate-grade MCTs in areas where wide surgical excision is not possible is a combination of surgery and RT. The veterinary literature has established that the complementary use of surgery to achieve clinical stage 0 disease (i.e., microscopically incomplete margins) and external-beam RT is associated with long-term control. Two-year control rates of 85% to 95% can be expected for low- or intermediate-grade stage 0 tumors.105,117,146–148 Some authors advocate prophylactic irradiation of cytologically negative regional lymph nodes (prophylactic nodal irradiation [PNI]).114,117,118,149 Due to the generally low risk of postsurgical metastasis in low- to intermediate-grade tumors, PNI is probably unwarranted in this group of patients, and at least one study has demonstrated no advantage in terms of disease-free or overall survival when PNI is employed in this group of dogs148; however, in MCTs at high risk for metastasis, PNI may provide improvement in outcome over local site irradiation only.114,149 The last option for low- or intermediate-grade MCTs in areas where wide surgical excision is not possible is a combination of surgery and chemotherapy (discussed later). There are now several published studies that have demonstrated a low rate of recurrence in dogs with incompletely excised MCTs that receive some form of chemotherapy postsurgery.150,151 Although not considered optimal, this approach can be used in cases in which RT is unavailable or unaffordable. Regardless of the local therapy chosen, dogs with low- and intermediate-grade tumors should be reevaluated regularly for local recurrence and possible metastasis. Local site and regional lymph node evaluation, complete physical examination, and aspiration of any new cutaneous masses or enlarged lymph nodes are performed at these intervals. More complete staging, including abdominal US, should be included if the dog has an MCT with negative prognostic indicators. For cases in which planned curative excisional surgery is unsuccessful and histologic margins are incomplete, further local therapy is warranted. A second excision of the surgical scar with additional wide margins should be performed if possible (Figure 20-6) or adjuvant RT can be used in cases in which re-excision is not an option. Not all MCTs with surgically incomplete margins will recur; in some studies, only 20% to 30% of MCTs with histologically confirmed incomplete margins recurred.84,152 Although recurrence rates vary by study, several studies have demonstrated increased recurrence rates and/or decreased overall survival in dogs with incompletely resected MCTs.28,71,73,113 Figure 20-7 summarizes the treatment recommendations for clinical stage 0 and I, histologically low- or intermediate-grade MCTs. Alternative local therapies for MCTs have been reported and include hyperthermia in combination with RT,153 intralesional brachytherapy,154,155 photodynamic therapy,156,157 intralesional corticosteroids,158 cryotherapy, and electrochemotherapy.159–161 Although some have advocated the use of intralesional deionized water at the site of an incompletely removed MCT, clinical data indicate that this approach is not effective at preventing local recurrence and should therefore not be used.162–167 It is important to note that none of these alternative local therapies are as thoroughly investigated, clinically effective, or practical as surgery, RT, or a combination of the two. Finally, despite its common use, there is no information available to suggest that adjuvant corticosteroid therapy is beneficial in cases of individual intermediate-grade MCTs that have been either excised completely or treated with local RT postsurgery.
Mast Cell Tumors
Canine Mast Cell Tumors
Incidence and Risk Factors
History and Clinical Signs
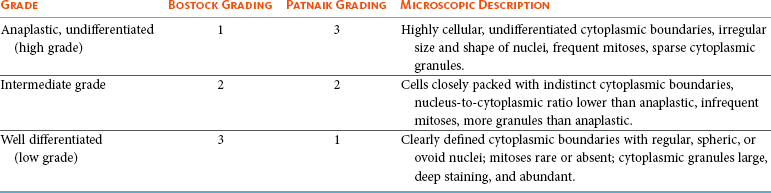
Prognostic Factors
Factor
Comment
Histologic grade
Strongly predictive of outcome. Dogs with undifferentiated tumors typically die of their disease following local therapy alone, whereas those with well-differentiated tumors are usually cured with appropriate local therapy.
Clinical stage
Stages 0 and I, confined to the skin without local lymph node or distant metastasis, have a better prognosis than higher-stage disease.
Location
Subungual, oral, and other mucous membrane sites are associated with more high-grade tumors and worse prognosis. Preputial and scrotal tumors are also associated with a worse prognosis. Subcutaneous tumors may have a better prognosis. Visceral or bone marrow disease usually carries a grave prognosis.
Cell proliferation rate
MI, relative frequency of AgNORs, percentage of PCNA, or Ki67 immunopositivity are predictive of postsurgical outcome.
Growth rate
MCTs that remain localized and are present for prolonged periods of time (months or years) without significant change are usually benign.
DNA ploidy
There is a trend toward shorter survival times and higher-stage disease in dogs with aneuploid tumors.
Microvessel density
Increased microvessel density is associated with higher grade, a higher degree of invasiveness, and a worse prognosis.
Recurrence
Local recurrence following surgical excision may carry a more guarded prognosis.
Systemic signs
The presence of systemic illness (e.g., anorexia, vomiting, melena, GI ulceration) may be associated with higher-stage disease.
Age
Older dogs may have shorter median DFIs when treated with RT than younger dogs.
Breed
MCTs in Boxers (and potentially other brachycephalic breeds) tend to be low or intermediate grade and are thus associated with a better prognosis.
Sex
Male dogs had a shorter survival time than female dogs when treated with chemotherapy.
Tumor size
Large tumors may be associated with a worse prognosis following surgical removal and/or RT.
c-kit mutation
The presence of an activating mutation in the c-kit gene is associated with a worse prognosis.
Diagnostic Technique and Work-Up
Treatment
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Mast Cell Tumors