Pamela L. Ruegg, Ronald J. Erskine, Consulting Editors Dawn E. Morin Specialized mammary glands to feed progeny are a defining characteristic of mammals, but the milk of several breeds of cattle, goats, and sheep has such excellent nutritional qualities, dairy herds are bred to produce milk for human consumption. Mammary gland diseases in these animals thus have important repercussions not only for the animals’ health but also for human well-being. The global dairy industry has changed dramatically in the past quarter century. Volatile milk prices, increasing herd size, reliance on hired labor, more complex feeding and housing systems, and increased pressure for environmental stewardship have fostered greater diversity among dairy enterprises. Bovine mastitis is the most common and economically important disease of dairy herds in developed countries1–3 and hinders realization of optimum farm productivity, milk quality, and supply of dairy foods. Mastitis remains the most common cause of antimicrobial drug therapy for cows on U.S. dairy farms.4 An important and often unrecognized obstacle to implementation of mastitis control practices is the behavior and attitude of farmers and employees.5 Veterinarians can play a critical role in controlling this disease as part of a quality milk team that targets drug use, environment, milking practices, and the epidemiology of mastitis within each dairy herd. Successful implementation of quality milk programs within dairy herds will increase productivity for dairy producers, supply milk processors with superior raw milk, offer consumers better-quality dairy foods, improve cow health and well-being, establish the dairy industry as advocates for antimicrobial stewardship, and promote a global sustainable market for U.S. dairy products. Decreased milk production in affected mammary glands is the primary economic loss associated with mastitis. Inflammation causes pathologic changes in milk-secreting epithelial cells that results in decreased functional capacity. Depending on the type of pathogen, losses may continue into subsequent lactations. Most infections result in local inflammation, or relatively mild clinical cases.6,7 However, chronic infections can lead to agalactia in the affected quarter, or in the case of severe clinical cases, profound systemic involvement that occasionally results in death. Somatic cells in milk are indicators of leukocytosis and primarily due to infection of the mammary gland. Bulk tank somatic cell counts (BTSCCs) strongly correlate with the proportion of infected cows within a dairy herd8 and are an indication of milk quality. Substantial economic loss may be incurred when regulatory or processor limits for BTSCCs are exceeded. Many milk processors routinely monitor BTSCCs, and dairy producers may be paid considerable monetary premiums for low BTSCCs or be penalized for high BTSCCs. Premium and penalty programs vary widely by location, but in some cases, penalty payments or unearned premiums account for the greatest loss associated with subclinical mastitis.9 Milk from cows with subclinical mastitis is not usually withheld from the bulk tank and may result in increased BTSCC and reduction in milk quality. Proteolytic and other changes in milk due to subclinical mastitis adversely affect organoleptic qualities and shelf life of milk and milk products. BTSCCs greater than 100,000 cells/mL are associated with decreased productivity per cow, shorter shelf life of fluid milk, and reduced yield of products like cheese.1,10,11 Individual cow milk yield is estimated to decrease 0.5 to 0.6 kg/day for each twofold increase in SCC over 50,000 cells/mL.12,13 However, the magnitude of the milk decrease relative to SCC increase has been reported to be higher in later lactation and older cows.13 Thus, a cow with subclinical mastitis and a SCC of 800,000 cells/mL is potentially losing 2 to 2.5 kg of milk each day relative to having a SCC of 50,000 cell/mL. High-BTSCC milk also has a greater risk of condemnation because of violative antimicrobial residues.14–16 In a retrospective study, the rate of antibiotic residue violations increased as herd SCC increased, and the relative risk of antibiotic residue violations (compared to herds with SCC < 250,000 cells/mL) was 2.4, 2.8, and 7.1 times greater for herds with SCCs of over 400,000, over 550,000, and over 700,000 cells/mL, respectively.14 Cows with chronically increased SCC caused by intramammary infections (IMIs) are often culled prematurely as a consequence of decreased milk production. The cost of premature culling depends on the perceived value of the cow, market prices for culled cows and replacement heifers, and the potential value of the replacement heifer.9 Cows with average SCCs above 700,000/mL were twice as likely to be culled than cows with SCCs under 250,000/mL, especially in herds with low BTSCCs.17 Cows that are not culled may be segregated or require use of milking procedures that increase labor costs. For example, cows with chronic IMI caused by Staphylococcus aureus or Mycoplasma may have to be housed separately and milked last. The incidence of clinical mastitis averages between 15% and 25% of lactations, although some herds may have rates greater than 45%.18–20 Estimates of lost milk production from a case of clinical mastitis over an entire lactation range from 110 to 552 kg, depending on the parity and stage of lactation when the case occurred.21 Further losses from clinical mastitis can result from subsequent repeated episodes during the same or later lactations. In a study of seven New York dairies, milk losses in the first 50 days following onset of clinical mastitis were 304, 128, and 92 kg for cases caused by gram-negative, gram-positive, and other pathogens, respectively.22 Clinical mastitis also results in additional economic losses beyond milk production. In a 1700-cow dairy, cows with clinical mastitis lost 341 kg of saleable milk, compared to projected yield, during the first 60 days after case onset.23 Nearly 75% of the milk loss was discarded because of treatment, with the remainder accounted for by lost production. Cows in their second or later lactation or more than 150 days in milk incurred greater losses, although the type of causative pathogen was not associated with differences in losses.23 The average total costs per case (in U.S. dollars) of gram-positive, gram-negative, and other pathogens were estimated to be $133, $211, and $95, respectively.24 Premature culling resulting from unresolved clinical mastitis cases or subsequent suboptimal milk yield of the affected cow may increase these costs to as much as $600 per case.25 The occurrence of both subclinical and clinical mastitis has been associated with reduced reproductive performance. In a preliminary field study, cows were almost two times more likely to have altered interestrus intervals following an episode of clinical coliform mastitis compared to herdmates without clinical mastitis.26 Conception rates for cows with clinical mastitis between 3 weeks before and 5 weeks after insemination have been reported to be lower compared to cows without clinical mastitis.27,28 Clinical mastitis due to gram-negative bacteria has a more detrimental effect on the probability of conception than clinical mastitis caused by gram-positive bacteria or other organisms.28 Additionally, the days to first service, services per conception, and the days to conception were all increased in cows with clinical mastitis as compared to non-mastitic cows.29 Cows with a case of clinical mastitis in the first 45 days of gestation are nearly three times more likely to abort within the next 90 days than non-mastitis cows.30 Cows with increased SCC (>150,000 cells/mL) also have decreased conception rates compared to cows with lower SCC, and as the SCC increases the odds of pregnancy decreases.31,32 Inflammatory mediators released in response to IMI likely play a role in oocyte and embryonic degradation.33,34 For example, lipopolysaccharide endotoxin in cattle induces a prolonged release of inflammatory mediators including PGF2α, leading to uterine smooth muscle contraction and luteolysis, a gradual decline in plasma progesterone, and thus abortion, especially in the first trimester of pregnancy.33 The total cost of mastitis is impossible to quantify accurately, varies over time, and is herd dependent. Nationally, the cost of mastitis has been estimated at $200/cow/yr, or $1.5 to $2 billion.9,35 Costs associated with implementation of mastitis control programs can be substantial. In one survey the cost of mastitis prevention accounted for 50% of the cost for preventing all diseases.36 Return on investment in mastitis control is more important than the absolute cost and ranged from −$20 to $275/cow/year in a review of 9 studies.37 However, this return depends on the prevalence and type of mastitis in the herd, existing control measures, and producer compliance.38 Although the costs incurred by producers to control mastitis are significant, the benefits of reducing mastitis include improved cow health and productivity, a superior raw milk supply for processors, better quality and safer dairy foods for consumers, and a more sustainable global market for dairy products. Cattle have a single udder composed of four independent mammary glands that are referred to as quarters. Suspensory ligaments attach the udder to the pelvic bone and abdominal muscles of the ventral body wall. The four quarters are independent and have no direct connections among them.39 Each mammary gland consists of a teat with a single opening, small cisterns (also called sinuses) in the teat and base of the gland, a system of ducts, and secretory tissue. The secretory tissue is organized into lobes composed of lobules containing hundreds of microscopic alveoli lined by mammary epithelial cells that synthesize and secrete milk.40 Nutrients required for milk synthesis are delivered to each mammary gland by the circulatory system and transported into epithelial cells or directly into the alveolar lumen. Milk components (e.g., casein, lactose, fat) are synthesized within epithelial cells and secreted into the alveolar lumen, where they combine with other constituents to form milk. Production of milk is continuous, and during the interval between milkings, about 20% of the milk accumulates in the larger spaces of the udder like the teat cistern, gland cistern, and large ducts (cisternal fraction). However, about 80% of the milk remains fixed by capillary forces within the alveoli (alveolar fraction), and removal of this fraction is dependent upon achieving successful milk ejection (milk letdown).41 Milk letdown occurs in response to tactile stimulation of teats, which triggers a neurohormonal reflex, causing the pituitary gland to release oxytocin into the bloodstream.41 Milk is ejected from the alveoli into the cisterns when the surrounding myoepithelial cells contract after binding oxytocin. Milk flows through the teat orifice after resistance of the teat canal (also called the streak canal) is overcome owing to pressure of milk in the teat, suckling by offspring, or through the process of milking. Activation of the sympathetic nervous system during periods of stress or excitement can inhibit oxytocin release from the pituitary gland or oxytocin binding to myoepithelial cells, thus preventing milk ejection.42 The teat canal and surrounding musculoelastic tissue provide the primary physical barrier to microbial invasion and also prevent milk leakage between milkings.43,44 The teat canal is a narrow, longitudinally folded cylinder lined with stratified squamous epithelium. The tortuous shape of the canal provides physical protection against infection, as does protein-rich keratin, which is continually produced by the epithelial cells and lines the canal. Lack of keratin in the teat canal greatly increases the risk of IMI, because keratin physically plugs the teat canal, traps invading microbes, and contains bactericidal fatty acids and proteins.45–47 Hyperkeratosis of the teat end, trauma-associated abrasion of the teat orifice, or teat damage from ineffective pulsation, excessive milking vacuum, or poorly fitting teat cup liners can also increase the risk of IMI.48–50 Because of the importance of the teat in mammary gland defense, dairy producers should routinely monitor teat condition and use a combination of genetic selection and management practices to promote teat health and sound conformation of the udder. However, it must be emphasized that cows that are housed on wet or soiled bedding or have udders and teats exposed to excessive water during milking preparation are at high risk for IMI, despite good teat end health.51–53 Additionally, lack of aseptic preparation of teat ends before cannula insertion or use of multiple-dose drug preparations that are not intended for intramammary infusion can result in outbreaks of infection caused by opportunistic pathogens.54 Preventing exposure to pathogens is the foundation of controlling infectious disease, including mastitis. When exposure to pathogens is sufficient to traverse the teat canal and multiply in milk, the ensuing host immune system response initiates inflammation of the mammary gland, which is recognized as subclinical or clinical mastitis. If the innate and acquired immune systems effectively eliminate the invading microbes, the mastitis will be mild and transient. However, when defense mechanisms are compromised (e.g., during the periparturient period or following transportation and comingling of new animals in the herd) or when the pathogen expresses virulence factors that resist phagocytosis or intracellular destruction, severe or chronic mastitis may develop. The intensity of the inflammatory response determines whether mastitis is subclinical or clinical. With subclinical mastitis, the inflammatory process does not result in visible abnormalities in the milk, mammary gland, or cow, although leukocytosis and other soluble changes in milk occur. With clinical mastitis, milk from the affected quarter is visibly abnormal, the gland may undergo marked inflammation, and the cow may exhibit signs of systemic illness. Although mastitis can result from trauma, the vast majority of mastitis is caused by IMI via the teat canal. As stated earlier, limiting exposure to pathogens at the teat end remains the primary means to prevent infection, but mastitis will occur even in well-managed herds. If invading pathogens overcome the teat canal barrier and gain entry into the gland, a series of innate host defenses help limit bacterial growth. Since bovine mastitis is essentially a disease of bacterial (and occasionally mycotic or algal) pathogens, the primary effectors of mammary immunity are polymorphonuclear neutrophils. However, as in other tissues, these phagocytes rely on a complex system of soluble and cellular mediators that recognize pathogen presence and subsequently recruit and activate phagocytes to eliminate the microbes. This system is called the innate immune system. Soluble factors include complement, lactoferrin, and acute-phase proteins (e.g., mannose-binding lectin, C-reactive protein). Lactoferrin is an iron-binding glycoprotein produced by mammary epithelial cells and found in neutrophil granules.55 By sequestering iron, lactoferrin prevents multiplication of iron-dependent microorganisms like coliform bacteria.56 Mannose-binding lectin and C-reactive protein will bind to molecules specific to pathogen cell walls and help activate the complement cascade.57 Innate host defenses differentiate host tissue from pathogens by recognizing molecules that are universal to many microbes but not present on the body’s own cells.58 These pathogen-associated molecular patterns (PAMPs) are recognized and selectively bound by serum proteins and receptors in host cells called pathogen recognition receptors (PRRs).58 Toll-like receptors (TLRs) are an important class of PRRs that reside both on cell surfaces and within host cells. These receptors invoke gene expression and release of inflammatory cytokines (e.g., interleukins [ILs], tumor necrosis factor [TNF]-α) from the host cells and act as the critical link between recognition of foreign agents and initiation of the host response.58,59 Examples of specific pathogen targets bound by TLRs include peptidoglycan and lipoteichoic acid of gram-positive bacteria (TLR-2), gram-negative lipopolysaccharide (LPS [TLR-4]), bacterial flagellin (TLR-5), and bacterial DNA as CpG oligonucleotides (TLR-9).60 Much of the local inflammation and systemic signs (when they occur) associated with mastitis are due to this response. Numerous cell types, including epithelial and endothelial cells, macrophages, and other phagocytic cells, possess TLRs that can contribute to an inflammatory response.61 The inflammatory response is further modulated by activation of eicosanoid (arachidonic acid) metabolites such as prostaglandins and leukotrienes, as well as endocrine and adipose mediators.62 Milk from healthy mammary glands contains a low concentration of cells, the majority of which are macrophages. However, when microbial ligands (molecules) are detected by TLRs on macrophages and epithelial cells, these cells release proinflammatory cytokines like IL-1, IL-6, and TNF-α.58 These mediators cause vasodilation and expression of adhesion molecules on endothelial cells, which in turn triggers an influx of neutrophils.60,63 Circulating blood neutrophils must adhere to the vascular endothelium before migrating into the milk. Vascular neutrophils loosely bind and roll along the endothelium, using a surface adhesion molecule (CD62L or L-selectin).64 L-selectin is highly expressed on the surface of normal circulating neutrophils, whereas similar molecules, E-selectin (CD62E) and P-selectin (CD62P), are expressed on vascular endothelial cells at the site of inflammation.64,65 Neutrophil CD62L is responsible for making initial contact between fast-flowing blood neutrophils and the vascular wall, which rapidly slows neutrophil movement in small postcapillary vessels, whereas endothelial CD62E and CD62P direct slow-moving neutrophils to the specific site of inflammation. Additional proinflammatory mediators like IL-8, leukotriene B-4, and complement component 5a serve as neutrophil chemotactic agents and promote expression of surface receptors on endothelial cells.64 This in turn causes neutrophils to express the β2-integrin adhesion complex CD11b/CD18. The CD11b/CD18 complex firmly anchors neutrophils to vascular and intercellular adhesion molecules.64,66,67 Once bound, neutrophils migrate between the endothelial and mammary epithelial cells into the milk (diapedesis), traveling along a chemotactic gradient to the site of infection.64 During infection, neutrophils often exceed concentrations of 1 million cells/mL. Both the speed of recruitment and extent of neutrophil influx into milk influence microbial clearance and are critical determinants of infection outcome.60,68–70 During migration and under the influence of proinflammatory cytokines (e.g., TNF-α and interferon [IFN]-γ), neutrophils are further activated to become focused killer cells.64 In this way, the leukocytes arrive at the infection site ready to recognize, phagocytose, and kill infecting pathogens. Phagocytosis is accomplished through a variety of specialized receptors on the surface of neutrophils, which are generally upregulated in response to the proinflammatory signals received during diapedesis and chemotaxis. Expression of CD14 receptors enables neutrophils to bind bacterial lipopolysaccharide (LPS) in the presence of LPS-binding protein (LBP); this binding facilitates nonopsonic phagocytosis of gram-negative bacteria.71 At the same time, soluble CD14 is shed into the milk, where it neutralizes free LPS and binds to epithelial cells, enhancing chemoattractant release.70 The most important neutrophil receptor for opsonic phagocytosis is the Fc receptor, which binds the Fc region of immunoglobulins (Ig), particularly IgG2 and IgM, enabling the phagocytosis of antibody-coated pathogens.72,73 Complement component 3b is also opsonic for bovine neutrophils.66 Receptor binding stimulates the neutrophil to extend pseudopods and engulf the adhered pathogen into a phagosome. The phagosome fuses with cytoplasmic secretory granules to form an intracellular vesicle (phagolysosome), where degranulation and microbial killing take place. Neutrophil granules contain cationic peptides called defensins that have broad-spectrum antibacterial and antifungal activities.55,60 These proteins, along with lactoferrin and hydrolytic enzymes, contribute to O2-independent killing of engulfed pathogens. To effectively respond to pathogen invasion, the lactating mammary gland of ruminants must overcome numerous deficits. The number of somatic cells in milk, which in lactating dairy cattle are primarily leukocytes, is typically less than 100,000 cells/mL in uninfected glands—about 100-fold lower than in blood. Concentrations of factors like lactoferrin and complement are also lower in milk relative to plasma.74 The ability of circulating macrophages in milk to reenter tissue for antigen presentation remains speculative, but compared to blood, macrophages and neutrophils in milk have decreased phagocytic ability.75,76 Neutrophils engulf fat globules and casein, which reduces subsequent pseudopod formation as well as intracellular killing capacity.70 Host-adapted pathogens (e.g., S. aureus) often have numerous virulence factors to help evade recognition and phagocytosis, which further compromises the ability of host defenses to eliminate the infection. If a pathogen survives innate host defenses, the specific (also called acquired) immune system is triggered. This branch of the immune system recognizes specific antigens of pathogens, and if repeated exposure occurs, an immunologic “memory” initiates a faster and more intense response with longer duration. As with neutrophils, mammary macrophages ingest and kill microorganisms.77 Macrophages appear to be particularly important in chronic IMI and during involution (dry-off) of the mammary gland. In addition to initiation of inflammation and pathogen killing, macrophages and dendritic cells play a key role in processing and presenting antigen to T lymphocytes. Antigen presentation is critical to activating T cells (CD4+), thus enabling cytokine secretion, activation of B lymphocytes, and cytotoxic (CD8+), suppressor, and memory functions.78 To date, the role of the CD4+ (helper), CD8+ (cytotoxic and suppressor), and CD17 (phagocyte agonist) subsets in bovine mammary immunity are best understood.79,80 B-lymphocytes are also critical for antigen processing and serve as precursors to immunoglobulin-producing plasma cells. Concentrations of immunoglobulin are low in normal milk but rise in response to IMI. This increase is partly a result of local Ig production but is mostly due to increased permeability of vascular endothelial cells and mammary epithelial cell tight junctions, which allows an influx of plasma Ig (and other plasma proteins) into milk. Influx of opsonizing Ig (IgM and IgG2) enables efficient phagocytosis of microorganisms by neutrophils.72,73,81 Nonopsonizing Ig (IgG1 and IgA in cattle) has other beneficial functions like neutralization of bacterial toxins, agglutination of bacteria, and prevention of bacterial adherence to epithelial cells. Immunoglobulins in milk also mediate antibody-dependent cell-mediated cytotoxicity by leukocytes.78,82 Mammary gland defense mechanisms are greatly reduced in the periparturient period, increasing the animal’s vulnerability to IMI. Indeed, both the incidence and severity of mastitis are greater in the periparturient period than during other periods of lactation.64,83–85 Phagocyte diapedesis and killing and lymphocyte mitogenic responses are markedly decreased during the periparturient period.79,82,86–88 Cows infected with S. aureus have a subpopulation of CD8+ lymphocytes within the mammary gland that suppress proliferation of CD4+ lymphocytes, and this class of CD8+ cells is preferentially trafficked into the gland during the postpartum period.80 The immunosuppression accompanying parturition is partly caused by increased circulating cortisol concentration, which impairs neutrophil margination and recruitment.64 Neutrophils of periparturient cows also undergo apoptosis more rapidly than neutrophils of cows in later lactation, and early onset of apoptosis reduces phagocytic and intracellular killing activity.70 Low circulating concentrations of insulin-like growth factor (IGF)-1 in periparturient cows may reduce neutrophil viability and impair cytokine secretion.85 Nutrition of cows during the transition period can also play a critical role in mammary immunity. Recently a critical link between negative energy balance and immune function in dairy cattle has been elucidated. Nonesterified fatty acids (NEFAs) are released from adipose tissue in response to negative energy balances that are typical for cows in the transition period from late gestation through peak milk production. Particularly before calving, cows with excessive negative energy balances and high serum NEFAs are more at risk for culling and diseases during early lactation.89,90 Additionally, elevated serum NEFAs lead to undesirable expression of proinflammatory mediators, adhesion molecules, and reactive oxygen species.91 The altered plasma fatty acid profile in cattle during periods of negative energy balance also changes the phospholipid content in membranes of endothelial and mononuclear cells.92 Fatty acids from membrane phospholipids are the precursors for numerous arachidonic acid metabolites, the control of which is critical for the magnitude of inflammatory responses. Antioxidant supplementation has also been shown to play an important role in mammary immune function. Vitamin E, selenoenzymes, and zinc-dependent superoxide dismutase protect cells and membranes from oxidative damage that could result from expression of reactive oxygen species during phagocyte killing.93 Compared to selenium-supplemented cows, cows that were selenium deficient showed reduced neutrophil phagocytosis and killing.94 Selenium deficiency also resulted in more severe outcomes in cows with coliform mastitis.95 In one herd, vitamin E supplementation above National Research Council (NRC) guidelines (4000 IU/day) to dry cows in the last 14 days before calving was reported to decrease clinical mastitis in the first 7 days of lactation.96 However, in a larger field study in five herds, feeding dry cows 3000 IU/day of vitamin E (which exceeds NRC recommendations) was found to increase both subclinical and clinical mastitis in the first 3 months after lactation.97 In this field study, antioxidant status in cows fed high levels of vitamin E varied, which may reflect the dependency of plasma α-tocopherol concentrations on lipid mobilization, which itself varies with individual cow energy balance.98 Although antioxidant supplementation is critical for mammary health, there is no compelling evidence to feed selenium, vitamin E, or any other nutrient of this type above NRC guidelines. Technically, mastitis can be defined as any inflammation of the mammary gland (including inflammation caused by injury), but almost all mastitis occurring in dairy cows is caused by pathogenic organisms that are mostly of bacterial origin but can also include yeasts, fungi, and algae.6 Like infections occurring in other tissues, mastitis begins with invasion of a pathogen, followed by a brief latent period, and then progression to either subclinical or clinical states or resolution of the infection as a result of the cow’s immune response.99 Detection of mastitis is almost never based on identifying the precise moment of infection but rather on observing the resulting inflammation. Recognition of mastitis is dependent on pathogen and cow characteristics that govern the magnitude of the immune response and the intensity and accuracy of the detection methods used on the farm. Subclinical mastitis is characterized by inflammation of the udder that is usually detected by enumeration of inflammatory cells in the milk. By definition, milk obtained from mammary gland quarters of cows experiencing subclinical mastitis appears normal but contains an abnormal number of somatic cells with or without the detectable presence of mastitis-causing bacteria.100 In most instances, detection of subclinical mastitis is based on evaluation of the SCC in an appropriate milk sample. The SCC can be measured using milk collected from individual quarters or composite milk samples that are a mixture of milk from all functional glands of an individual cow. Composite milk samples are routinely used in Dairy Herd Improvement Association (DHIA) programs for monitoring SCCs of individual cows. When composite SCC values are used, some subclinical infections will not be detected because the SCC of the composite milk will be reduced by dilution with milk from healthy quarters that contain few somatic cells.101 When cows are not routinely tested for individual SCC, veterinarians cannot determine important epidemiologic characteristics of subclinical infections (e.g., prevalence, incidence) necessary for implementing mastitis control programs. The distribution of cell types found in bovine milk varies depending on infection status and milk fraction.102 The SCC of healthy quarters is quite low and usually remains well below 100,000 cells/mL.103 However, based on detection of IMI (microbiologically positive milk samples), a threshold of less than 200,000 cells/mL is usually considered the optimal value to define a mammary quarter as healthy.100,104–106 Selecting the appropriate threshold for defining subclinical mastitis depends the goal of the control program. Lower thresholds will identify more animals with subclinical infections (increased sensitivity and fewer false negatives), whereas higher thresholds (increased specificity) will result in fewer false positives.106 Regardless of the threshold selected, reviewing the history of SCC values for a cow is much more informative than observing a single monthly value. A SCC above 200,000 cells/mL in milk does not mean bacteria will be recovered from milk samples obtained from the affected gland. The immune response reflected by the influx of leukocytes can be quite effective at reducing the number of bacterial colonies in milk, and about 10% to 25% of quarters that are above a threshold of 200,000 cells/mL will be apparently bacteriologically negative.100,104–107 When historically important contagious pathogens (e.g., Streptococcus agalactiae and S. aureus) have been controlled, the rate of apparent “false negatives” (inability to recover bacteria from quarters that exceed the SCC threshold) is often even greater. These culture-negative samples do not always indicate that the quarter is cured, but merely that the immune response has reduced the number of bacteria to below normal laboratory detection limits. It is useful to educate clients that about 30% to 50% of milk samples collected from mammary glands with high SCCs will be culture negative, so sufficient samples should be collected to ensure meaningful diagnostic test results. The duration of the subclinical phase of IMIs will vary depending on etiology. In most herds, a greater proportion of subclinical mastitis is caused by gram-positive than by gram-negative pathogens (Table 36-1). Usually, gram-negative opportunistic pathogens like Escherichia coli have a shorter subclinical phase than gram-positive pathogens like S. aureus or most IMIs caused by Streptococcus spp.108,109 In general, some 25% to 35% of cows experiencing chronic subclinical mastitis caused by gram-positive pathogens may eventually exhibit clinical symptoms.110 For opportunistic environmental pathogens, the likelihood of a clinical episode occurring in a cow with subclinical IMI varies among pathogens. For example, about 50% of IMIs caused by streptococci progress to clinical signs, but over 80% of coliform infections will exhibit clinical signs.108 TABLE 36-1 Common Mastitis-Causing Bacteria* * Results of selected studies that describe the distribution of bacteria recovered from milk obtained from cows with clinical and subclinical mastitis in modern dairy herds located in developed countries. † Results characterized as contaminated and mixed infections were excluded. ‡ NR indicates that the study did not report that outcome. § Contaminated cases are included. It is important to diagnose the prevalence and incidence of subclinical infections within a dairy herd. Cows with subclinical mastitis infections are known to produce considerably less milk and maintain a reservoir of infection that can result in increased exposure to potential pathogens of otherwise healthy cows.111 Subclinical infections that persist for long durations are especially costly. For example, cows that maintain subclinical mastitis across the dry period (SCC > 200,000 cells/mL at the last test of the completed lactation and first test of the subsequent lactation) have been shown to produce 9.1 kg (20 lb) less milk at their first DHIA test day.106 In diagnostic laboratories, SCCs of milk can be determined using methods like direct microscopic observation or, more commonly, electronic counting of dyed cells in either fresh, preserved, or frozen milk.112,113 About half of U.S. dairy farms subscribe to monthly DHIA testing and have access to SCC values determined using composite milk samples collected from individual cows.114 SCC values are not usually normally distributed, and many DHIA centers report the SCC using a linear score transformation that is calculated using a simple formula (LS = log2 [SCC/100] + 3). This transformation results in a normal distribution of the values and a linear association of the transformed data with estimates of losses in milk yield that are caused by subclinical infections.115 Review of monthly summarized SCC values at the herd level is a critical component of udder health programs and should be part of routine veterinary activities on dairy farms. Laboratory-based methods for identification of subclinical mastitis are considered to be accurate, but because they are removed from the farm, they are sometimes criticized as too slow for making cow-side decisions. To overcome this hurdle, a number of indirect measures of SCC have been developed. The California Mastitis Test (CMT) is an example of an indirect test that has been used for more than 50 years to help guide mastitis control programs.116 The CMT was developed to test milk from individual quarters but has also been used on composite milk samples and bulk milk samples.116 The CMT reagent is a mixture of a detergent plus bromcresol purple (used as an indicator of pH). The degree of reaction between the detergent and the DNA of cell nuclei is a measure of the number of somatic cells in milk. The CMT test is generally read using a five-point scale that begins with “negative” (SCC range of 0 to 200,000 cells/mL) and “trace” scores (SCC range of 150,000 to 500,000 cells/mL) and ends with a maximum score of 3 (SCC > 5,000,000 cells/mL). The CMT was developed during an era when many cows had very high SCCs as a result of chronic subclinical infections caused by S. agalactiae and S. aureus. Adoption of standardized best management practices has decreased the prevalence of these pathogens,117 and SCC values of cows infected with other pathogens are typically much less. Although the CMT continues to have considerable utility, it is important to note that even “trace” scores indicate the presence of subclinical mastitis, and the test may be difficult to read when used on milk obtained from cows in the first few days of lactation.118,119 In addition to increased SCC, a number of changes in milk composition are due to the immune response to infection. Compositional changes include reduced casein, lactose, and α-lactalbumin concentrations; an influx of sodium, chloride, and plasma proteins; increased proteolytic and lipolytic activity; an increase in lactoferrin concentration and enzyme activities; and a rise in pH.120 Many of these changes can be used as indicators of subclinical mastitis. Among the more common milk tests are those that detect albumin, sodium, chloride, lactose, or adenosine triphosphate (ATP) concentration; N-acetyl-β-d-glucosaminidase (NAGase) activity; antitrypsin activity; or pH. Plasma tests detect α-lactalbumin, casein, or lactose concentration.120,121–125 Milk concentrations of the acute-phase proteins amyloid A and haptoglobin are also used to distinguish healthy quarters from those with subclinical mastitis.121,125 With increases in diagnostic technology and increased emphasis on production of high-quality milk, considerable research has been focused on producing tools for continuous in-line monitoring of milk quality. The increased use of automatic milking systems (robotic milking) has led to the search for automated methods that can consistently and accurately detect abnormal milk.126 Current testing technology generally gives accurate results, but the ultimate value of the test depends on the value of the management decision that is based on the test result; veterinary practitioners should continuously review how such tests can be best used on dairy farms. Clinical mastitis is usually defined as the production of abnormal milk with or without secondary symptoms. Detection of clinical mastitis is based on observation of foremilk prior to attaching the milking unit, but the working definition of clinical mastitis varies greatly among farm personnel. Many farms do not routinely remove or observe foremilk of all cows, so detection of clinical mastitis is limited to cases with more severe signs.114,127 To better understand the incidence of mastitis, veterinarians should ask milking technicians to describe their individualized definition of clinical mastitis symptoms. Clinical signs of mastitis result from inflammation and may include abnormal appearance of milk (presence of clots or serum); swelling, redness, or edema of one or more quarters; or severe systemic signs such as anorexia, fever, or agalactia. Compared to subclinical mastitis, a much greater proportion of clinical cases are caused by gram-negative pathogens (see Table 36-1). Like other bacterial diseases, many cases of mastitis occur as a syndrome, with the infected quarters alternating between a clinical state and a subclinical state.108 The disappearance of clinical signs simply indicates that inflammation has decreased and does not always coincide with bacteriologic cure.128 The ability to achieve a bacteriologic cure depends on the etiology, case severity, variation in immune response among cows, efficacy of the treatment protocol, and promptness of initiating treatment.129 Recording the standardized severity scores can help veterinarians to better define the pattern of clinical mastitis on individual farms.128,130–132 One severity scoring system uses a three-point scale that combines the appearance of milk with the progression to additional clinical signs. A score of 1 (mild mastitis) = abnormal milk only; 2 (moderate mastitis) = abnormal milk and abnormal udder; 3 (severe) = systemic symptoms.128 This system is practical, simply recorded, and can be an important way to assess detection intensity. In most herds, the distribution of clinical mastitis by severity is approximately 40% to 50% mild cases, 40% to 50% moderate cases, and 5% to 15% severe cases.7,128,130,132,133 Veterinarians are rarely asked to treat mild and moderate cases of clinical mastitis and therefore may not understand the true incidence of clinical mastitis on many dairy farms.134 Veterinarians need to promote the use of clinical mastitis records that allow them to monitor the occurrence of mild and moderate cases and also the ability to evaluate outcomes of mastitis treatments. Treatment and prevention of mastitis is based on understanding the etiology of mastitis. As dairy farms increase in size, an increasingly diverse group of pathogens have been associated with the occurrence of mastitis.7,134–136 Clinical signs may be suggestive of some pathogens, but it is impossible to diagnose the etiology based on the appearance of the milk, gland, or animal.137,138,139 Some practitioners erroneously believe they can recognize coliform infections based on severity of the symptoms, but researchers have consistently shown that the majority of clinical cases caused by coliform bacteria present with only mild or moderate symptoms,7,131,133 and misdiagnosis of coliform infections is common.139 Submission of an aseptically obtained milk sample to a laboratory qualified to perform appropriate microbiological or molecular testing is necessary to determine etiology. Milk samples may be individually collected from affected quarters (quarter milk samples) or combined from all four glands into a single vial (composite milk samples). Quarter milk samples collected from affected glands are more sensitive than composite samples in detecting bacteria from subclinical infections, but the sensitivity of composite samples increases with the number of infected glands per cow.140,141 Sampling the cow on more than one occasion also increases the likelihood of detecting glands that are intermittently shedding some pathogens.142 The concentration of bacteria per milliliter of milk varies among pathogens. Some pathogens (e.g., S. agalactiae and most other Streptococcus spp.) consistently shed large numbers of bacteria in milk. For these pathogens, composite milk samples may be acceptable because concentrations of bacteria will more consistently exceed the microbiological detection limit.143 For other pathogens (e.g., S. aureus and Mycoplasma spp.) composite samples will often result in apparently culture-negative samples, because these pathogens are often shed in fewer numbers, and dilution with milk from healthy quarters will result in samples that contain less than the limit of detection in most laboratories (100 colony-forming units [CFU]/mL of milk).144 Composite milk samples are often used in an attempt to reduce the cost of routine screening of high-risk animals for the presence of existing subclinical infections with potentially contagious mastitis pathogens. Pooling 5 to 10 aseptically collected milk samples is another strategy used in some herds to further reduce costs. When either of these strategies is used, it is important to recognize that the potential for false-negative outcomes is increased. The sensitivity of composite milk samples for detection of S. aureus and Streptococcus dysgalactiae was estimated to be about 73% to 77%, in contrast to about 60% to 62% for Streptococcus uberis and coagulase-negative staphylococci (CoNS).145 In all instances, sensitivities increase as the number of infected mammary gland quarters increases.145 When using pooled or composite samples, veterinarians should routinely review cow histories to identify cows with increased SCCs that are typical of cows with subclinical infections. Mastitis is usually caused by a single type of bacteria, and the National Mastitis Council (NMC) has developed standards that specify that recovery of more than two types of bacteria from an aseptically collected milk sample is indicative of contamination.6 Before collection of milk samples, technicians should be trained in aseptic collection techniques. To maximize the possibility of recovering bacteria from the sample, milk should be collected after the teats have been prepared for milking (application of pre-milking disinfectant and drying) but before the units are attached.146 Sampling before milking is usually easier because cows are generally more likely to stand still and there is less pressure from farm workers to release the cows. Before obtaining the sample, the udders should be clean, and dry-wiping the udder with a paper or cloth towel is often helpful in reducing the possibility of contamination. Milk should be collected in sterile vials by technicians who are wearing disposable nitrile or latex gloves and using a standardized process. After thoroughly drying the teat using a cloth or paper towel, the teat end should be vigorously scrubbed using 70% ethyl or isopropyl alcohol. If composite samples are collected, a separate alcohol swab must be used for each teat. Sanitation is not complete until the surface of the swab remains clean after it is used. The cap should be removed from the sample vial without touching the inside, and it should be held so that the inner surface faces down. Milk from the teat to be sampled can be directed at an angle into the sampling vial. A sample size of 3 to 5 mL is usually adequate. The cap should be immediately replaced after the sample is obtained. When sampling multiple teats, the far teats should be scrubbed before the near teats, and the near teats sampled first to avoid contamination by the sampler’s arm or hand. Samples must be immediately cooled, refrigerated at 4° C, held on ice, or frozen until cultured. Failure to use aseptic technique or mishandling after collection can produce erroneous results. If samples are to be submitted to a diagnostic laboratory, they should be submitted within 24 hours of collection. If samples cannot be processed within 24 hours, they should be frozen until transported to the lab. Freezing for periods of less than 2 weeks has minimal effects on recovery of most mastitis-causing bacteria but can reduce recovery of Mycoplasma spp.147 When proper sampling and laboratory techniques are used, recovery of bacteria from milk samples is highly specific for mastitis. However, microbiological examination of milk obtained from glands affected with clinical or subclinical mastitis is not very sensitive.148 Failure to recover bacteria from a milk sample obtained from a gland with a high SCC does not usually mean that bacteria are not the causative agent for mastitis. When a single milk sample is obtained from dairy cattle exhibiting clinical or subclinical mastitis, approximately 35% to 50% of milk samples will be culture negative (see Table 36-1).7,117,149 Repeated sampling of affected quarters can increase the probability of reaching a diagnosis.142,144 Standardized laboratory methods for identifying mastitis pathogens have been well defined by the NMC.6 In general, technicians inoculate media that contain nutrients required for bacterial growth, and the inoculated media are placed in an incubator that contains the appropriate atmosphere and temperature to encourage growth of the target organisms. After 24 to 48 hours, the plates are examined for growth, and the bacteria are identified using a variety of specific test methods that depend on observable bacterial characteristics. In most mastitis laboratories, 10 to 100 µL of each milk sample are inoculated onto a portion of a blood agar plate (5%). The inoculum volume determines the lower limit of detection. For example, one colony observed on a plate inoculated with 0.01 mL (10 µL) is equivalent to roughly 100 CFU/mL of milk, whereas one colony observed using a 0.1-mL (100-µL) inoculum is equivalent to about 10 CFU/mL. The use of larger (0.05- or 0.10-mL) volumes of inocula increases recovery rates for pathogens shed in low concentrations (E. coli, S. aureus).151 Other ways to increase recovery are preculture centrifugation,150 incubation,141,152,153 or freezing152 of the milk, or a combination of these methods. The optimal method is pathogen dependent. Augmented methods are most useful for detecting subclinical S. aureus infections and reducing false-negative results for clinical mastitis samples. After inoculation, agar plates are incubated at 37° C (98.6° F) and observed for growth at 24 and 48 hours. There is no absolute definition of IMI, but the presence of at least 100 to 300 CFU/mL is usually required to define an infection. Bacterial identification is based on phenotypic characteristics of the colonies and the result of additional laboratory tests. S. aureus is usually differentiated from other staphylococci based on a positive coagulase reaction and other typical phenotypic characteristics (e.g., hemolysis).6 Streptococci are usually identified using the Christie-Atkins-Munch-Petersen (CAMP) test and esculin reactions. When milk samples originate from cases of clinical mastitis, MacConkey agar is usually also inoculated to facilitate rapid identification of gram-negative, lactose-fermenting organisms (coliforms). Additional biochemical tests are required for final identification at the species level. Identification of Mycoplasma spp. requires use of media containing specific nutrients not found in general media and incubation in a CO2-enhanced environment.
Mammary Gland Health

Mammary Gland Health of Dairy Cattle
Economic Impact of Dairy Cattle Mastitis
Anatomic Structures of the Bovine Mammary Gland
Immunologic Response to Intramammary Infection
Mammary Inflammation.
Specific Immune Responses.
Defense Mechanisms in the Periparturient Period.
Diagnosis and Detection of Mastitis
Definitions
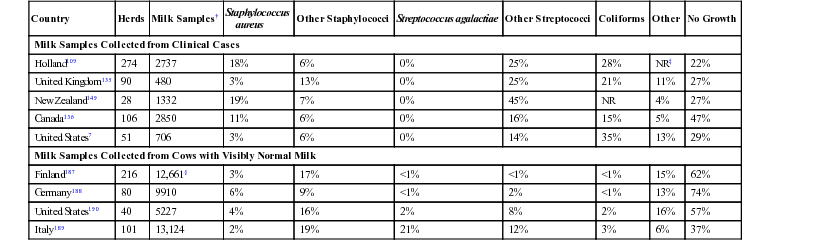
Country
Herds
Milk Samples†
Staphylococcus
aureus
Other Staphylococci
Streptococcus agalactiae
Other Streptococci
Coliforms
Other
No Growth
Milk Samples Collected from Clinical Cases
Holland109
274
2737
18%
6%
0%
25%
28%
NR‡
22%
United Kingdom135
90
480
3%
13%
0%
25%
21%
11%
27%
New Zealand149
28
1332
19%
7%
0%
45%
NR
4%
27%
Canada136
106
2850
11%
6%
0%
16%
15%
5%
47%
United States7
51
706
3%
6%
0%
14%
35%
13%
29%
Milk Samples Collected from Cows with Visibly Normal Milk
Finland187
216
12,661§
3%
17%
<1%
<1%
<1%
15%
62%
Germany188
80
9910
6%
9%
<1%
2%
<1%
13%
74%
United States190
40
5227
4%
16%
2%
8%
2%
16%
57%
Italy189
101
13,124
2%
19%
21%
12%
3%
6%
37%
Identification of Etiologic Agents
Milk Samples Collected From Individual Animals.
Laboratory Methods.
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


