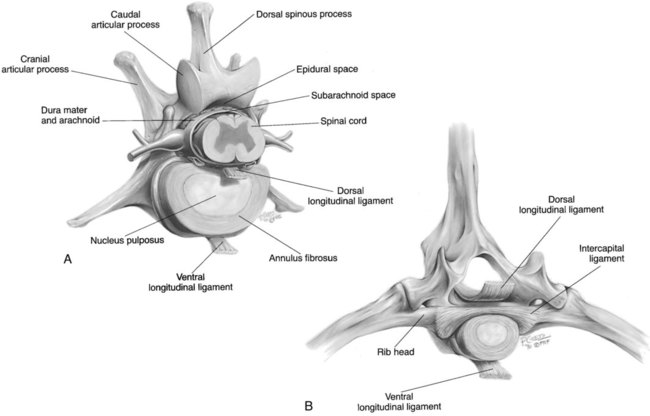
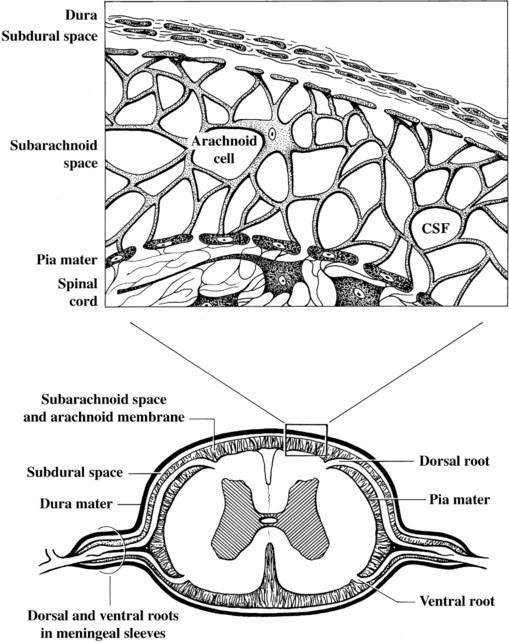
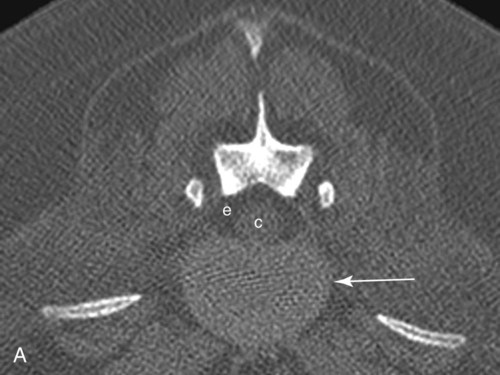
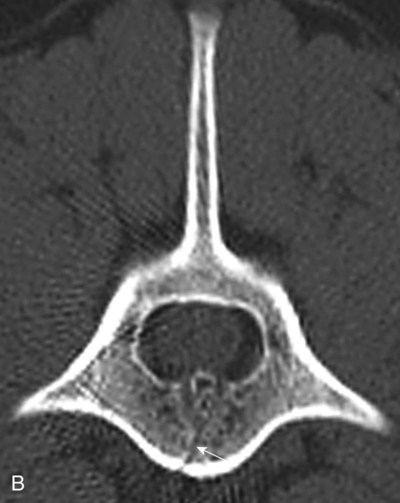
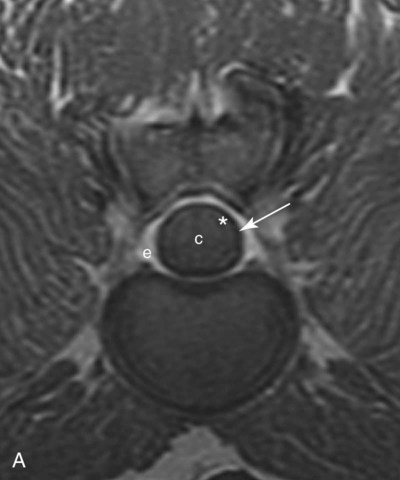
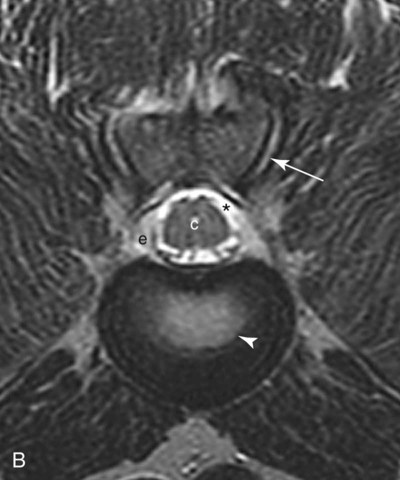
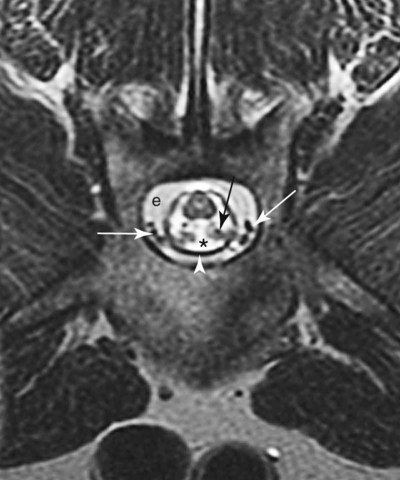
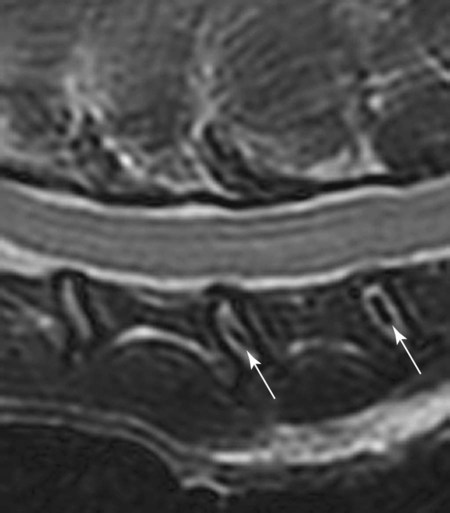
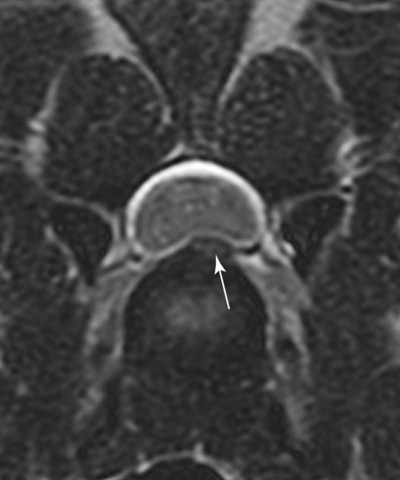
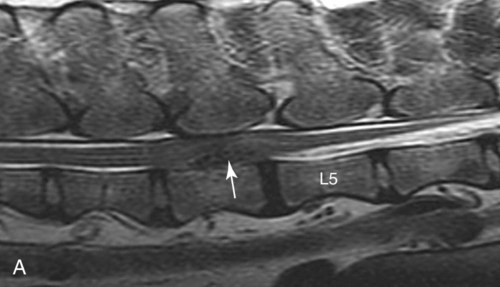
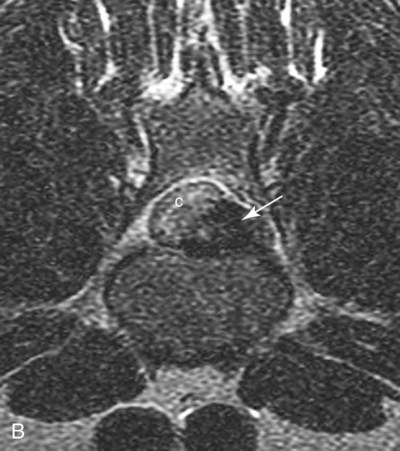
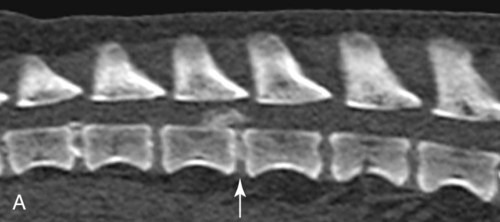
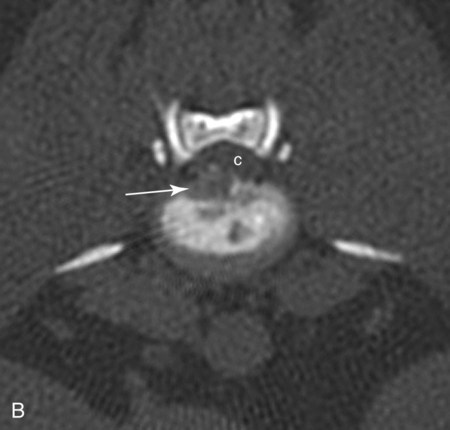
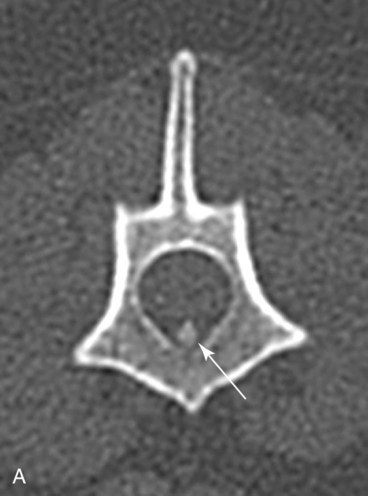
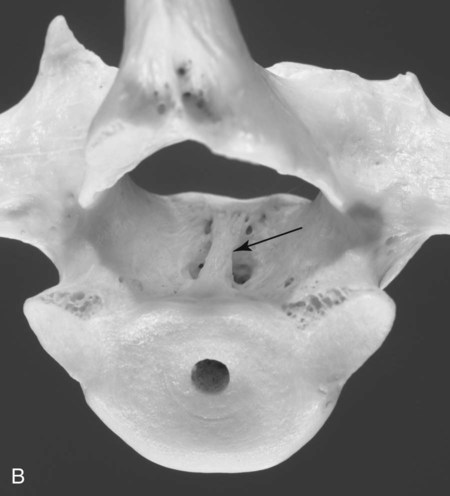
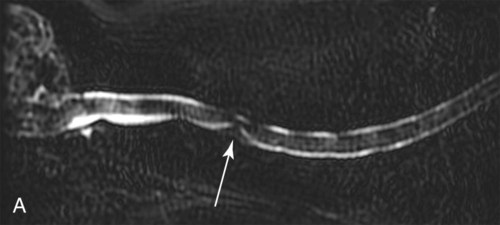
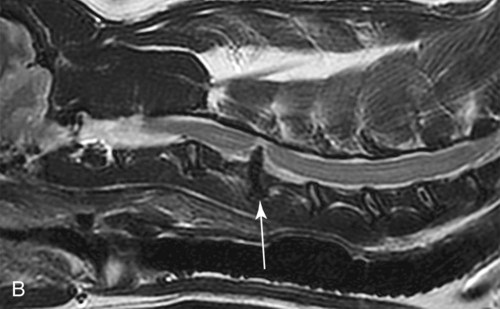
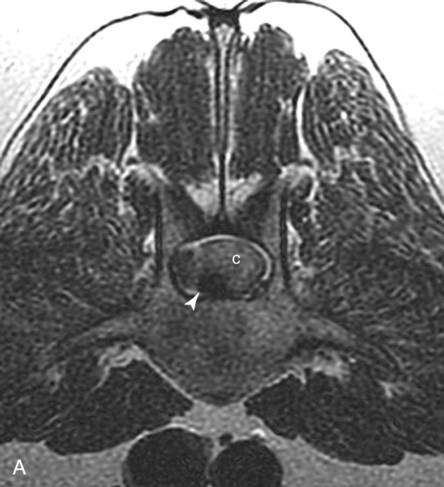
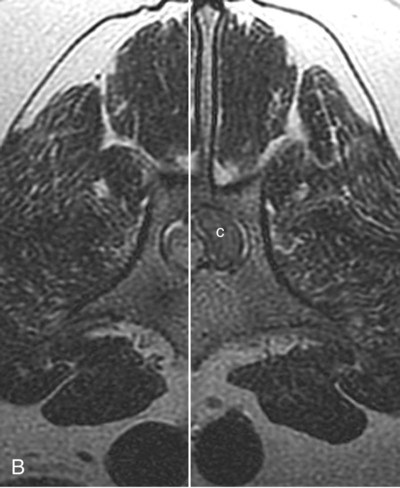
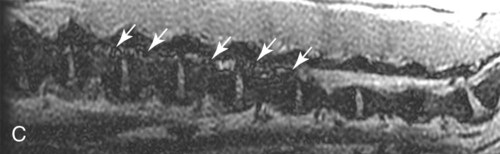
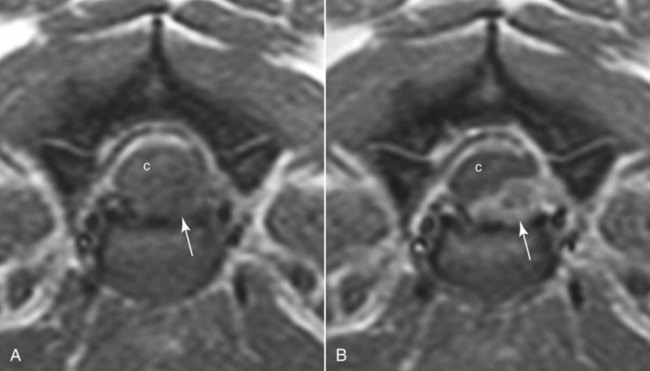
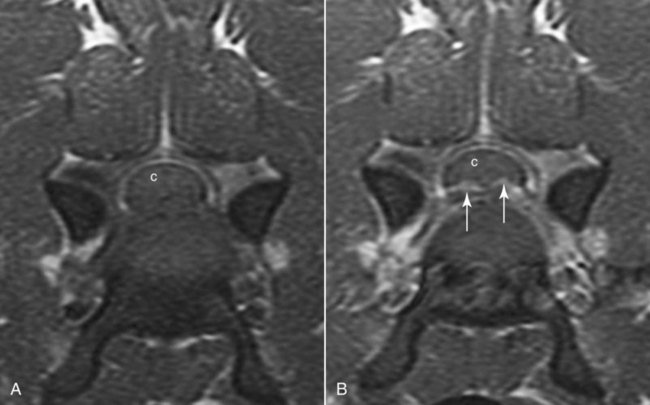
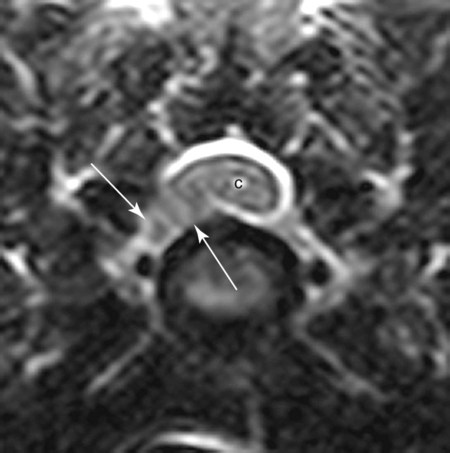
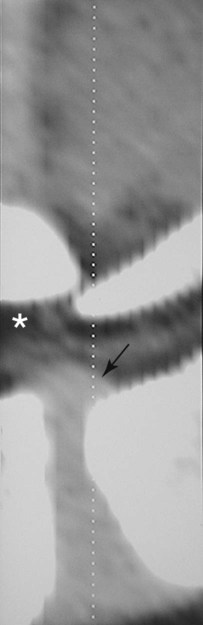
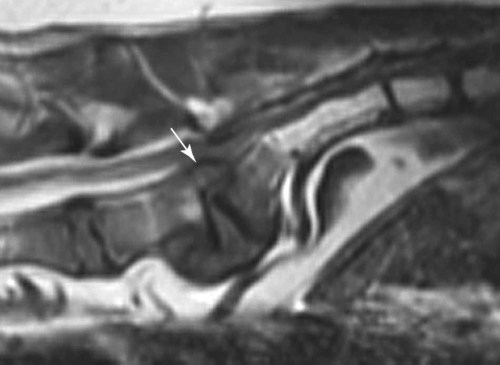
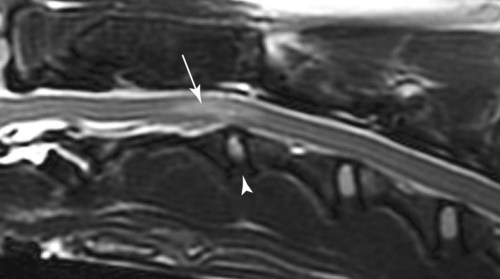
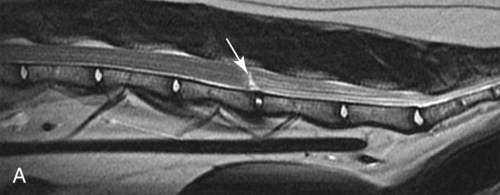
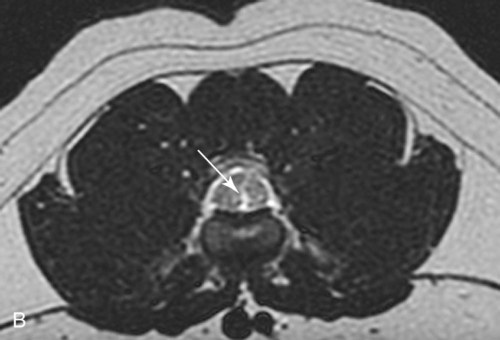
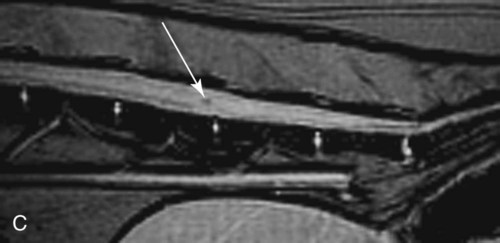
Computed tomography (CT) and magnetic resonance (MR) imaging are used routinely in the investigation of spinal diseases in the dog and cat.1–2 They are both superior to radiography in the diagnosis of many spinal conditions. MR imaging has a better overall diagnostic sensitivity than CT and can be used to diagnose virtually all conditions of the spine. However, there are patients in which CT is still the preferred method, such as for safety reasons, for example, vertebral trauma caused by a gunshot wherein metallic foreign material is present or for the diagnosis of subtle vertebral subluxation or fractures. Although a number of spinal conditions can be diagnosed with radiographs (e.g., discospondylitis), CT and MR imaging may still be warranted to better assess the extent of the condition, which may be important for diagnostic or therapeutic purposes. Earlier diagnosis of some conditions may also be permitted by CT or MR imaging. A schematic representation of the components of the vertebrae and main ligaments of the spine is shown in Figures 12-1 and 12-2. The longitudinal ligaments of the vertebral column provide dorsal and ventral support for the intervertebral discs.3 The dorsal longitudinal ligament lies on the floor of the vertebral canal. In the cervical region, it is wide and thick, which explains why lateral disc extrusion leading to radiculopathy, or root signature, is more common in the cervical region than other spinal regions. In the thoracolumbar area, the dorsal longitudinal ligament is thinner and centered on the midline allowing dorsal protrusion of disc material leading to spinal cord compression. The intercapital ligaments are short, transverse fibrous bands that lie ventral to the dorsal longitudinal ligament, joining left-to-right rib heads between T2 and T113; they therefore buttress the dorsal part of the annulus fibrosus cranial to T11, reducing the probability of dorsal disc protrusion in this region. The ventral longitudinal ligament spans the ventral surface of the vertebral column.3 The yellow ligaments (ligamentum flavum), also called interarcuate ligaments, are loose, thin, elastic sheets that bridge the space between the arches of adjacent vertebrae.3 The spinal cord and spinal nerve roots lie in the vertebral canal, which is formed by the juxtaposition of all the individual vertebral foramina of the spine. The spinal cord begins at the foramen magnum and terminates caudally at the conus medullaris, around L6. The actual termination is caudal to L6 in smaller breeds of dogs and cats, whereas it is cranial to L6 in larger canine breeds.4 Normal segmental widening of the spinal cord is present at the cervical and lumbar intumescences, and this should not be mistaken for pathologic cord swelling.3 Spinal cord segments and vertebrae have the same numeric designation, with the exception of spinal cord segment C8, but the location of each spinal cord segment is usually cranial to the corresponding vertebral segment.3 The ratio between the diameter of the spinal cord and the diameter of the vertebral canal varies between dog breeds, being higher in dachshunds and in other chondrodystrophic breeds in general versus nonchondrodystrophic breeds.5 The spinal cord is surrounded by the meninges. The pia mater is the innermost meningeal layer; it is firmly attached to the spinal cord and highly vascular.6 The arachnoid membrane is next, and the dura mater is the outermost meningeal layer. The arachnoid and dura are attached closely to each other by a layer of dural border cells.6 The subarachnoid space lies between the arachnoid and pia mater and contains cerebrospinal fluid (CSF) and arachnoid trabeculae that attach the arachnoid to the pia mater. The epidural space is peripheral to the dura mater and contains fat and the internal vertebral venous plexus.6 The nerve roots exit through the intervertebral foramina. The collection of spinal nerve roots in the lumbosacral area is known as the cauda equina. CSF is found in the spinal subarachnoid space, which begins at the foramen magnum, where it communicates with the intracranial subarachnoid space, and ends caudally at the filum terminale near the lumbosacral junction. CSF is also found in the central canal of the spinal cord, which communicates with the fourth ventricle cranially and terminates caudally blindly at the conus medullaris or in some subjects is continuous with the lumbar subarachnoid space (see Figs. 12-1 and 12-2).3 On CT, cortical bone is thin and of uniformly high attenuation with smooth margins. Cancellous bone has a lacy or honeycomb appearance (Fig. 12-3). All components of the vertebra (laminae, pedicles, body, foramina, basivertebral venous canal, bony processes) are well visualized with CT. At the midportion of the body, the basivertebral venous canal is visible as a Y-shaped lucency, and a small dorsally projecting ridge of bone is seen within the ventral floor of the vertebral foramen at this site (see Fig. 12-3; see also Fig. 12-10).7 The dorsal articular process diarthrodial joints have thin, smooth subchondral bone, and the articular processes are separated by a hypoattenuating space corresponding to synovial fluid and articular cartilage. On transverse images centered at the disc spaces, the combined margins of the dorsal aspect of the annulus of the intervertebral disc and dorsal longitudinal ligament and ventral aspect of the annulus and ventral longitudinal ligament appear as elliptical soft tissue attenuating structures. Occasionally the interarcuate ligament (ligamentum flavum) is seen as a curvilinear opacity spanning the dorsal laminae and blending with the joint capsule of the articular process joints. The ventral, dorsal, interspinous, and intertransverse ligaments are not resolved from surrounding structures. Epidural fat is hypoattenuating with respect to the soft tissue structures of the vertebral canal. The combination of the spinal cord, blood vessels, subarachnoid space with CSF, and meninges form a round to oval soft tissue attenuating structure in the middle of the vertebral canal that is surrounded by a rim of hypoattenuating epidural fat (see Fig. 12-3). Nerve roots can be seen as circular or linear soft tissue attenuating structures depending on their orientation in the scan plane. When CT is performed after myelography, the thecal sac is hyperattenuating, allowing for more accurate differentiation among intramedullary, intradural, and extradural conditions. CT myelography can be achieved by injecting iodinated contrast medium in the subarachnoid space, at 25% of the regular myelographic dose.8 This allows excellent delineation of the spinal cord and is useful in characterizing compressive lesions or spinal cord atrophy. The normal cross section of the cervical spinal cord is kidney shaped and slightly larger at C6-C7. In the thoracic area, there is a dramatic reduction of the spinal cord diameter, and it is also more round.8 On MR imaging, cortical bone appears as a black shell around the vertebra.9 On T1-weighted images, epidural and paraspinal fat are hyperintense to the spinal cord, whereas the intervertebral discs have medium signal intensity (Fig. 12-4).9 The hyperintense epidural fat provides contrast for other spinal structures. The spinal cord, nerve roots, and bone marrow are isointense or slightly hypointense to the intervertebral discs. A low-signal shell around the cord represents a combination of CSF in the subarachnoid space, chemical shift artifact, and meningeal structures.9 The dorsal and ventral longitudinal ligaments, as well as the ligamentum flavum, are visible only in the intervertebral areas where they are separated from the bone; they are of low signal intensity.9 The joint capsule and synovial fluid of the articular process joints are not visible. The internal vertebral venous plexus consists of two symmetric hypointense oval structures, sharply marginated, located ventral to the spinal cord in the mid-body regions and diverging slightly from the ventral location at the level of the intervertebral disc spaces. On T2-weighted images, normal intervertebral discs have high signal because of high water content in the nucleus pulposus and inner portion of the annulus fibrosus (see Fig. 12-4).9–10 The epidural and paraspinal fat are still hyperintense, and the spinal cord and nerve roots are hypointense (Fig. 12-5). The bone marrow is hypointense compared with fat and is isointense or hypointense to the spinal cord. Depending on the amount of T2 weighting, the CSF in the subarachnoid space forms a thin hyperintense shell around the cord.9 Sequences with a very heavy T2 weighting, such as single-shot fast spin echo (SSFSE), enhance the signal from CSF while suppressing the signal from background tissue, giving a natural myelographic effect.11 Intervertebral discs are made of a peripheral annulus fibrosus and a central nucleus pulposus.10 The disc lies in close contact with the cartilaginous vertebral end plates with fibers from the nucleus pulposus and annulus fibrosus being interwoven with the collagen fibers of the cartilaginous end plates and bony trabeculae.9 Both the annulus and the nucleus are made of fibrocartilage but differ in the amount of collagen and ground substance.12 There is more collagen and less ground substance in the annulus than in the nucleus. The ground substance is composed of hyaluronic acid and glycosaminoglycans that hold water because of their strong negative charge.12 A normal nucleus pulposus has a gelatinous consistency. Water, bound to large proteoglycan molecules, is the principal component of the nucleus pulposus (80% to 88%).13 The normally very bright signal of the nucleus pulposus on T2-weighted images is caused by the long T2 relaxation time of water in the nucleus (see Fig. 12-4).10 Apart from trauma causing extrusion of nondegenerated disc material (see specific paragraph later), degeneration of the intervertebral disc occurs before clinically significant intervertebral disc disease (IVDD).10 Degenerative IVDD was first classified by Hansen.14 Hansen type I IVDD is herniation of the nucleus pulposus through annular fibers with subsequent extrusion of nuclear material into the vertebral canal. Type I IVDD is more common in chondrodystrophic dogs. The degenerative process leading to type I herniation involves shifting concentrations of glycosaminoglycan, loss of water and proteoglycan content, and increased collagen content. The disc becomes more cartilaginous, and its nucleus becomes more granular, often mineralizes, and loses its hydroelastic shock-absorbing qualities (Fig. 12-6). The degenerative process starts early in life, and herniation is observed in younger animals (2 to 7 years, with a peak incidence at 4 to 5 years). With more detailed imaging of the patterns of disc herniation that resulted from MR imaging, IVDD can be divided into several categories based on appearance more than physiopathology:15 • A bulging disc corresponds to circumferential extension of the disc beyond the margins of the vertebral end plates. • A protruding disc is the partial extension of the nucleus and inner annulus through disrupted fibers of the outer annulus but without complete rupture of the outer annulus (Hansen type II) (Fig. 12-7). • An extruded disc represents true herniation (Hansen type I), where portions of the nucleus and inner annulus traverse through all the layers of the outer annulus and form a focal extradural mass deviating epidural fat and, depending on the size of the herniation, the subarachnoid space and spinal cord (Fig. 12-8). This herniated disc material can detach completely from the disc where it originates and form a free extradural mass, with various degrees of dispersion.15 • Nondegenerative traumatic disc herniation: If a disc with a normally hydrated nucleus pulposus is placed under extreme stress, the dorsal annulus fibrosus may rupture, and some of the normal jellylike nucleus pulposus may explode into the vertebral canal and cause spinal cord contusion. Because the nucleus material has no degenerative changes and is normally hydrated, it diffuses in the peridural fat, leaving only the secondary changes attributable to acute spinal cord contusion with little or no spinal cord compression.13 Several terms have been used to describe this syndrome, including traumatic disc prolapse, traumatic disc herniation, disc explosion, noncompressive nucleus pulposus extrusion, Hansen type III disc disease, high-velocity-low-volume disc disease, traumatic intervertebral disc extrusion, missile disc, and acute noncompressive nucleus pulposus extrusion.13,16 Such herniations are associated most commonly with trauma such as a road traffic accident or running into an obstacle but can also be associated with vigorous exercise.17 CT of the spine for assessing the intervertebral disc should be obtained using thin slices (1 to 2 mm), low pitch (<2), and a medium-frequency image-reconstruction algorithm.18 When CT is used in the assessment of the spinal cord, subarachnoid injection of iodinated contrast medium is often performed concurrently because the spinal cord is often not clearly visible on CT images. Obviously, the use of CT with subarachnoid contrast medium invalidates the potential advantage of diagnosing disc herniation without the side effects associated with myelography, although the dose of contrast medium for CT myelography is less than that for myelography. CT without intrathecal injection of iodinated contrast medium for detection of IVDD has been evaluated in chondrodystrophic dogs, which are likely to have some degree of mineralization of the herniated material.19 On CT, vertebrae can be identified as thoracic or lumbar without difficulty based on the presence of ribs or transverse processes. The location of any particular image can therefore be identified by counting sequential vertebrae or by using scout images depicting slice location. Acquiring lateral and ventrodorsal scout images will reduce the risk of missing a vertebral malformation that could affect accurate localization of individual disc spaces at surgery. This is important, because 10% of dachshunds have transitional anomalies that lead to deranged vertebral anatomy.20 If the scout images are not of sufficient quality to identify vertebral abnormalities, it may be useful to obtain a routine survey radiograph before performing the CT scan. In chondrodystrophic breeds, CT is excellent at identifying herniated disc material and provides accurate information as far as location, laterality, and extent of spinal cord compression (Fig. 12-9). In chondrodystrophic dogs, acute herniation leads to either a large focal amount of markedly hyperattenuating material (approximately 200 HU) compressing the spinal cord or to more diffuse and less hyperattenuating material (approximately 60 HU) causing less severe spinal cord compression. In the latter instance, the material in the vertebral canal corresponds to areas of epidural hemorrhage. In chronic disc herniation, the attenuation of the herniated material is higher than that of acutely herniated disc material (approximately 700 HU) because the herniated material continues to mineralize after acute herniation.19 Some pitfalls exist when interpreting CT images. For example, a round focus of mineralization is seen occasionally on transverse images dorsal to the vertebral bodies. This focus has been thought to represent mineralization of the dorsal longitudinal ligament,19 which fans out to a thin sheet over the intervertebral disc space but narrows to a circular midline structure over the vertebral body. However, this focal hyperattenuation is more likely a transverse image of the small bony ridge between the dorsal vertebral foramina (Fig. 12-10). This hyperattenuation can be mistaken for disc material. Although conventional CT is adequate for the diagnosis and localization of mineralized Hansen type I disc extrusions in chondrodystrophic breeds, CT myelography is often necessary for diagnosis if no lesion is identified in a chondrodystrophic dog, if plegia is present because of concurrent extradural compression and spinal cord swelling, or if the dog is nonchondrodystrophic.21 Although there may be no improvement in patient outcome when using MR imaging for diagnosis of IVDD,22 it is the method of choice for presurgical diagnosis because it is noninvasive and provides superior anatomic detail as well as potential prognostic indicators (e.g., myelomalacia). Diagnosis of IVDD is made from MR images when the following signs are seen: • Extradural compression of the spinal cord centered at the level of an intervertebral disc (Fig. 12-11; see Fig. 12-8): This is recognized by a loss of signal from the epidural fat and a change in the shape of the spinal cord, as well as a change in the normal ovoid shape of the disc, and is best appreciated on transverse images (see Fig. 12-7). • Degeneration of the disc (loss of T2-hyperintense signal from the disc). • Narrowing of the intervertebral disc space: This is usually best evaluated on sagittal T1-weighted images.23 MR imaging is accurate in determining the location and craniocaudal length of extruded intervertebral disc material. T2-weighted images are more reliable in determining the extent of the compressive material.24 Heavily T2-weighted sequences, such as SSFSE, provide a myelographic effect by enhancing the signal of the CSF and suppressing the signal from other structures and can be used to quickly spot areas of cord compression based on identification of interruption and/or deviation of the CSF column (see Fig. 12-11).11 Epidural hemorrhage and inflammation associated with disc extrusion can be detected with MR imaging.25 In dogs with thoracolumbar or lumbosacral herniation, the incidence of such epidural changes is approximately 5%.25 These changes are more common in the caudal aspect of the lumbar spine and observed more commonly when there has been migration of disc material. The signal intensity of these epidural lesions is variable, appearing hyperintense to the spinal cord or heterogeneous on T2-weighted images, and hyper-, hypo-, or isointense to the spinal cord on T1-weighted images.25 This variation likely reflects changes in magnetic properties depending on the age of the hemorrhage and amount of disc-associated inflammation. Gradient recalled echo T2-weighted pulse sequences are sensitive to the paramagnetic properties of deoxyhemoglobin and methemoglobin, and hemorrhage or hematomas in the epidural space create hypointensities as a result of susceptibility artifact (Fig. 12-12).26 Contrast enhancement of epidural hemorrhage is common, although it is not associated histopathologically with actual inflammation. In some dogs these secondary epidural changes can mask the extruded disc material. Altogether, these features can cause erroneous diagnosis of a tumor, and this variability needs to be taken into account when interpreting MR images in dogs with acute neurologic presentation. There does not appear to be a difference in clinical outcome in dogs with epidural hemorrhage or inflammation compared with dogs without these changes.25 Regardless of whether epidural hemorrhage or inflammation is present, contrast enhancement of the compressive extradural disc material occurs in approximately 50% of dogs with disc extrusion (Fig. 12-13).25,27 Meningeal contrast enhancement adjacent to extruded extradural disc material also occurs in up to 40% of patients (Fig. 12-14, see page 202).27 Cervical IVDD accounts for 14% to 16% of intervertebral disc disease in dogs.28 Type I cervical IVDD is common in small-breed dogs, especially dachshunds and beagles, but is also seen in large-breed dogs, especially Labrador retrievers, German shepherd dogs, Rottweilers, and Dalmatians.29–30 In small-breed dogs, the most common sites of herniation are C2-C3, C3-C4, and C4-C5, whereas in large-breed dogs the most common sites are C4-C5 and C6-C7.29–30 The amount of compressive myelopathy occurring with cervical IVDD is typically less than with thoracolumbar disc herniation because the dorsal longitudinal ligament is wider in the cervical area than the thoracolumbar region. Cervical disc herniation also occurs most commonly in the dorsolateral direction, between the dorsal longitudinal ligament and vertebral venous sinus, thereby causing nerve root compression with root signature and cervical pain (Fig. 12-15, see page 202). Also, the cervical spinal cord occupies a smaller proportion of the vertebral canal so that the degree of compression caused by an equal volume of herniated disc material is less than in the thoracolumbar area.28 In the cervical area there is a correlation between the degree of spinal cord compression caused by disc herniation and the neurologic grade at time of imaging; however, there is no correlation with outcome after surgery.28 The most commonly affected discs in the thoracolumbar region are T12-T13 and T13-L1.31 In dogs with neurologic deficit caused by thoracolumbar disc extrusion (Hansen type I), there is no definitive association between the degree of spinal cord compression as defined on T2-weighted transverse images and the neurologic grade at presentation or outcome after surgery.31–32 However, the length over which the spinal cord is compressed is associated with the neurologic grade at presentation, but not with outcome.32 In dogs with paraplegia because of acute thoracolumbar disc extrusion, areas of spinal cord hyperintensity on T2-weighted series are often present (Fig. 12-16). The development of intramedullary T2 hyperintensity following disc herniation is associated with more severe neurologic deficits at presentation.25,27,27 Areas of intramedullary T2 hyperintensity that exceed the length of L2 on sagittal images have been associated with a poor outcome in approximately 50% of patients.33 This feature appears to be a better predictor of poor outcome than loss of deep pain perception33,34 and is independent from the severity of neurologic signs at presentation.32 There has been an anecdotal report of sacrocaudal disc extrusion causing tail pain and problems with defecation, with resolution of these clinical signs after surgical correction. MR imaging and CT can be useful in confirming the presence of extruded disc material in the sacrocaudal canal.35 Degenerative lumbosacral stenosis is an abnormal narrowing of the L5-S3 vertebral canal or intervertebral foramina, with compression of the cauda equina and/or its blood supply.36 Degenerative lumbosacral stenosis is characterized by intervertebral disc degeneration, intervertebral disc protrusion, bone proliferation on the vertebral end plates and articular processes, vertebral subluxation, and hypertrophy of the articular process joint capsule and interarcuate ligament (ligamentum flavum).36 CT and MR imaging are both excellent for characterizing degenerative lumbosacral stenosis in dogs.7,9,36–42 In dogs with clinical signs of cauda equina syndrome, CT changes include loss of epidural fat, increased soft tissue opacity in the intervertebral foramen, bulging of the intervertebral disc, spondylosis, thecal sac displacement, narrowed intervertebral foramen, narrowed vertebral canal, thickened articular processes, articular process subluxation, and articular process osteophytosis. On sagittal images, a step between the floor of the vertebral foramen of L7 and the floor of the vertebral foramen of S1 is often seen (Figs. 12-17 and 12-18).42 Enhancement of the soft tissue compressive material is often observed after injection of contrast medium, and this feature should not be interpreted as evidence of a tumor.36 Compression should be suspected in sites where there is increased perineural soft tissue material with absence of epidural fat.37,41 Interpretation of CT changes in the lumbosacral area must be made with caution, as some changes can be present in dogs free of clinical signs and therefore insignificant clinically. In particular, idiopathic lumbosacral stenosis and loss of perineural fat have been reported in dogs with no clinical signs.38 In addition, only slight to fair agreement was found when comparing results of CT or MR imaging of the lumbosacral spine to surgery.42 For both CT and MR imaging there does not appear to be an association between imaging findings and clinical outcome after surgery in working dogs with clinical signs of degenerative lumbosacral stenosis.36 Occasionally, disc extrusion occurs without preexisting degenerative disc disease when an otherwise healthy disc is subjected to a brief excessive force. Such extrusions occur typically during performance of strenuous exercise or following trauma. Names applied to this syndrome were outlined previously in the section “Classification of Intervertebral Disc Disease.”13,16–17,43–48 Typically, the condition is characterized by an acute, often asymmetric myelopathy that is nonprogressive after the first 24 hours.16 The clinical presentation is similar to primary ischemic myelopathy.16 On MR imaging, acute noncompressive intervertebral disc extrusion is characterized by the following:13,16,16 • Focal intramedullary T2 hyperintensity overlying an intervertebral disc. The area of maximal hyperintensity is usually just dorsal to the affected intervertebral disc but can sometimes be seen just cranial or caudal to the disc space. The longitudinal extension of the intramedullary hyperintensity is usually more focal than with ischemic myelopathy, which has a similar clinical presentation (Fig. 12-19).13,16 • This intramedullary lesion is usually isointense on T1-weighted images and does not enhance after gadolinium injection, although occasional focal precontrast T1-weighted hyper- or hypointensity and mild postcontrast enhancement may be present.13 • There is reduction in volume and signal intensity of the nucleus pulposus of the corresponding disc on T2-weighted images (Fig. 12-20; see Fig. 12-19). • A narrowed intervertebral disc space is seen on sagittal images. • There is extraneous material or signal changes within the epidural space dorsal to the affected disc space with absence of or minimal spinal cord compression. • A communicating T2-hyperintense tract can occasionally be seen traversing the dorsal annulus between the residual nucleus pulposus and the vertebral canal.13 The acutely extruded disc material most commonly damages the spinal cord through contusion without penetrating the dura, but occasionally it can cause a dural tear and penetrate the spinal cord.43,44,46–48 In these instances, a vertical intramedullary linear T2-hyperintense lesion crosses the spinal cord and is contiguous ventrally with the intervertebral disc space. Areas of hypointensity can be seen associated with that tract in gradient recalled echo images because of hemorrhage (see Fig. 12-20).46,48
Magnetic Resonance Imaging and Computed Tomography Features of Canine and Feline Spinal Cord Disease
Normal Appearance of the Spine on Computed Tomography and Magnetic Resonance Imaging
Normal Anatomy
Computed Tomography
Magnetic Resonance Imaging
Intervertebral Disc Disease
The Normal Intervertebral Disc
Classification of Intervertebral Disc Disease
Computed Tomography of Intervertebral Disc Disease
Magnetic Resonance Imaging of Intervertebral Disc Disease
Regional Characteristics of Intervertebral Disc Disease
Cervical IVDD
Thoracolumbar IVDD
Sacrococcygeal Disc Herniation
Lumbosacral Stenosis
Acute Noncompressive Intervertebral Disc Extrusion
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Magnetic Resonance Imaging and Computed Tomography Features of Canine and Feline Spinal Cord Disease