CHAPTER 7 Lymph Nodes
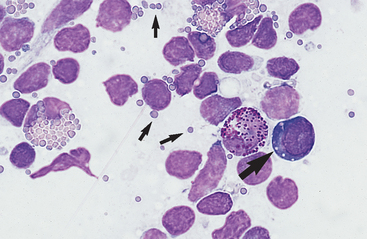
Cytology is a useful tool for evaluating peripheral lymphadenopathy. Cytologic examination of lymph node aspirates provides a quick, easy, and inexpensive way to gain insight into the cause of the lymphadenopathy without significant risk to the patient. Lymph node cells exfoliate easily, resulting in highly cellular aspirates that allow for reliable cytologic interpretation. Cytology may yield a definitive diagnosis (eg, lymphosarcoma, some infectious lymphadenopathies) or may indicate the process causing lymphadenopathy (eg, immune stimulation, purulent inflammation).
Lymphadenopathy can be localized or generalized and primary or secondary in nature. Causes of lymphadenopathy include hyperplasia, lymphadenitis (neutrophilic, purulent, eosinophilic, granulomatous), immune stimulation, lymphosarcoma, and metastatic neoplasia. An algorithmic approach to lymph node aspirate evaluation and interpretation is presented in Fig. 7-1.
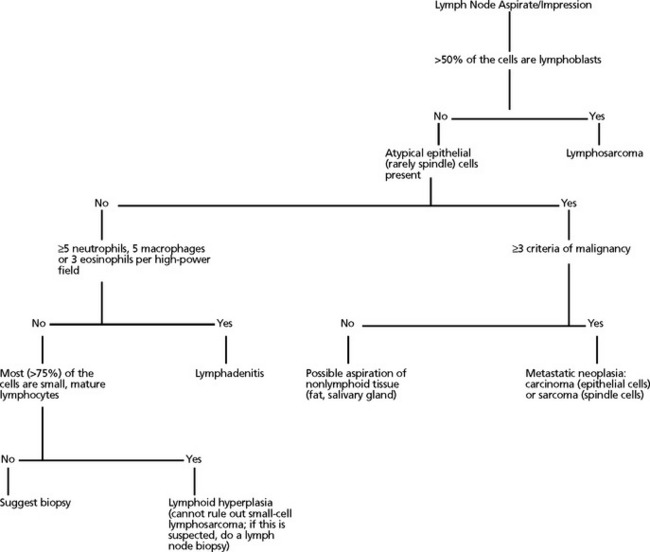
Fig. 7-1 Algorithmic approach to cytologic evaluation of lymph node aspirates.
(Courtesy Oklahoma State University, Clinical Pathology teaching files.)
Sample Collection and Preparation
When tissues are aspirated for cytologic evaluation, the tissue of least resistance is aspirated into the needle and/or syringe. If a vessel is ruptured and blood is aspirated, continued aspiration results in excessive blood contamination. This dilutes the sample and impairs cytologic evaluation. Therefore, once blood enters the syringe and/or needle, negative pressure should be released and the needle redirected, or the aspiration procedure should be repeated with a new syringe and needle, depending on the degree of blood contamination.1
When the sample appears in the hub of the needle or if the needle has been redirected three or four times but the sample is still not visible in the syringe or hub of the needle, negative pressure is released and the needle is withdrawn from the node. The needle is removed from the syringe and a few milliliters of air (2 to 4) is drawn into the syringe barrel. The needle is replaced on the syringe and the plunger is rapidly depressed, forcing some of the aspirated contents onto a clean, dry glass slide. The sample must be smeared into a monolayer without causing excessive cell rupture (Fig. 7-2). Because lymphocytes, especially lymphoblasts, are fragile and rupture easily, special care must be taken. A combination smear procedure is depicted in Fig. 1-2. This requires some technical skill but can be mastered easily with minimal practice.
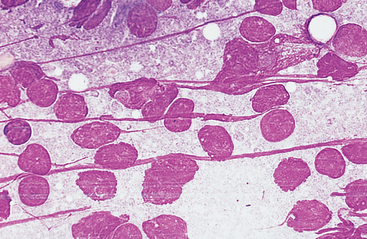
Fig. 7-2 Lymph node aspirate with all nucleated cells stripped of cytoplasm.
Nuclear chromatin of many cells forms strands across the slide. Smears with excessive cell rupturing cannot be evaluated cytologically. (Wright’s stain; original magnification 250X)
Nonaspiration Technique (Capillary Technique, Stab Technique): A 22-gauge needle is attached to a 3- to 10-ml syringe that has been prefilled with air. The node to be sampled is held firmly to aid penetration and to help direct the needle. The needle/syringe apparatus is grasped as if holding a throwing dart. The needle (attached to the syringe) is introduced into the mass. The needle is moved rapidly back and forth through the mass and along the same plane five to six times. There is no need to aspirate, since the cells are collected by shearing and capillary action. The needle is removed from the mass, and the material is expelled onto a clean glass microscope slide by rapidly depressing the plunger. Smears are made by one of the techniques described in Chapter 1.
Generally, one collects only enough material to make one smear. Therefore, the procedure should be repeated two or three times in different sites of the node or in different nodes to have adequate slide numbers and areas to evaluate.
Staining
The staining procedure recommended by the stain’s manufacturer should be followed in general but adapted to the type and thickness of smear being stained and to the evaluator’s preference. In general, the thinner the smear, the less immersion time needed in the stain. The thicker the smear, the more immersion time needed in the stain. For this reason, thick smears, such as smears of neoplastic lymph nodes, may require the recommended immersion time in stain be doubled or tripled. Each person tends to have different preferred staining characteristics. By trying variations in the recommended time intervals for the stains, the evaluator can establish immersion times to produce the preferred staining characteristics.1
Lymph Node Cell Types
Small Lymphocytes
Small lymphocytes are ≤9 μ in diameter (recognizably smaller than neutrophils) and have a scanty amount of clear to light blue cytoplasm that may contain a few azurophilic (red-purple) granules (Fig. 7-3). The nucleus is round to oval, usually indented, with dense clumps of nuclear chromatin in a coarse, smudged pattern. Nucleoli are not visible.
Medium Lymphocytes
Medium lymphocytes are 9 to 12 μ in diameter (about the same size as neutrophils), with a moderate amount of bluish cytoplasm that sometimes contains a few azurophilic granules. The nucleus is oval to irregularly shaped, with a stippled to granular chromatin pattern. Normal prolymphocytes may not have recognizable nucleoli or may have indistinct to prominent nucleoli. However, with prolymphocytic lymphosarcoma, nucleoli are usually single, large, and prominent. The presence of nucleoli in a nontraumatized lymphocyte indicates that it is either a prolymphocyte or a lymphoblast (large lymphocyte).
Large Lymphocytes (Lymphoblasts)
Lymphoblasts are larger than neutrophils, have an increased amount of bluish cytoplasm that may appear somewhat granular, and sometimes contains one to several azurophilic granules. A clear area in the cytoplasm representing the Golgi apparatus may be evident. The nucleus is oval, cleaved, notched, or irregular, with a finely stippled to granular chromatin pattern. Multiple small to large nucleoli are generally evident. While the cytoplasm is increased in volume, it typically is not recognizable all the way around the nucleus but tends to run confluent with the nucleus in one or more areas (see Figs. 7-9 and 7-10).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree