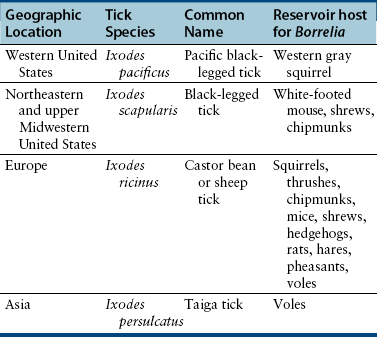
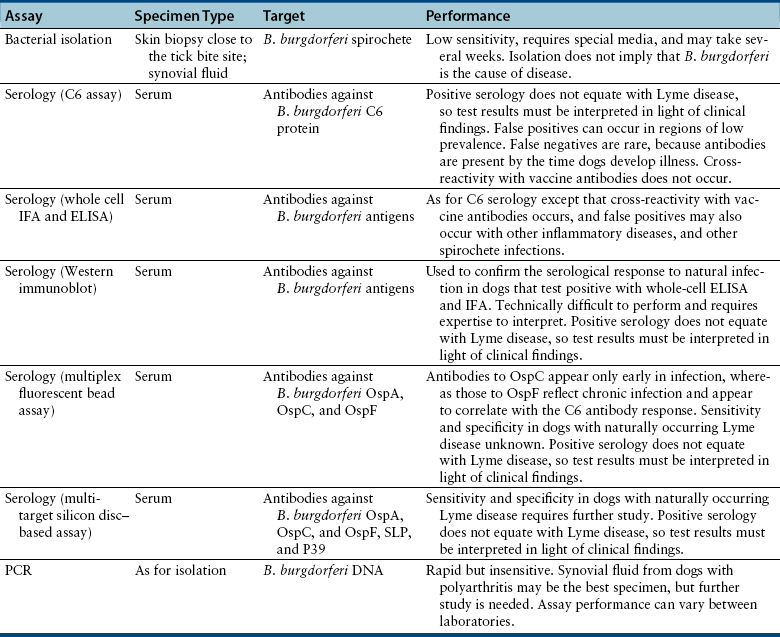
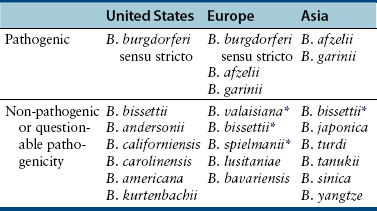
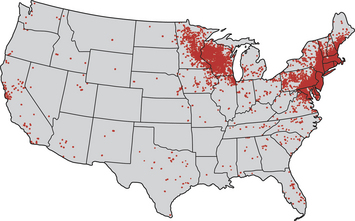
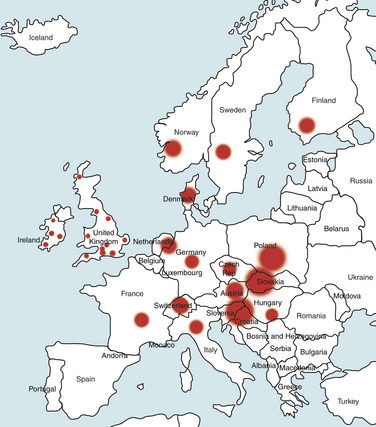
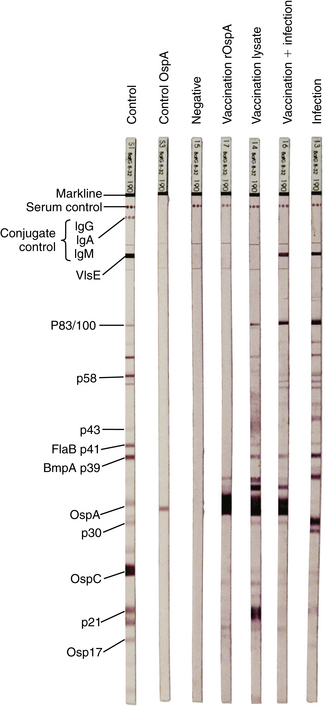
Chapter 51 Lyme borreliosis, or Lyme disease, is a vector-borne spirochetosis caused by the motile, corkscrew-shaped bacterium Borrelia burgdorferi sensu lato. Lyme disease occurs in North America, Europe, and Asia. It was named after Old Lyme in Connecticut, where clusters of the disease were first recognized in children with juvenile rheumatoid arthritis in 1976.2 However, chronic cutaneous manifestations of infection were recognized in human patients in Germany as far back as 1883.3 The spirochete was first detected in ticks by Burgdorfer in 1982.1 A variety of different mammalian hosts and humans may be infected. The disease has been increasingly recognized and is now the most common vector-borne infectious disease of humans in the United States and Europe. Reforestation of farmland and proliferation of deer and tick populations may have contributed to emergence of the disease in humans.4 Cats can be infected and seroconvert, but appear to be relatively resistant to development of disease.5 Dogs can develop fever, arthritis, and renal disease, but most infected dogs show no signs of illness. More than 15 different species of B. burgdorferi sensu lato have been described, some of which are nonpathogenic (Table 51-1). Borrelia turicatae, the cause of tick-borne relapsing fever in humans, has been detected in sick dogs from Texas.6 The DNA of Borrelia lonestari, which is thought to be nonpathogenic, has been detected in dogs from Arkansas.7 In North America, Lyme disease is caused by Borrelia burgdorferi sensu stricto, whereas in Europe and Asia, other species that belong to Borrelia burgdorferi sensu lato are more important, specifically Borrelia afzelii and Borrelia garinii. This may account for differences in clinical manifestations that occur in human patients in Europe compared with those in North America (see Public Health Significance, later). Some strains of B. burgdorferi appear to have increased virulence, such as B. burgdorferi OspC type A within the United States.8 TABLE 51-1 Pathogenic and Nonpathogenic Species That Belong to Borrelia burgdorferi sensu lato and Their Geographic Distributions ∗B. valaisiana and B. bissettii have been isolated from single cases of Lyme borreliosis; B. spielmanii has been detected in early skin lesions (Stanek G, Reiter M. The expanding Lyme Borrelia complex—clinical significance of genomic species? Clin Microbiol Infect 2011;17:487-493). B. burgdorferi sensu lato is transmitted by Ixodes ricinus- persulcatus complex ticks (Table 51-2). The geographic distribution of Lyme disease reflects that of the vector ticks as well as the competency of the reservoir hosts involved. In the United States, the vectors are Ixodes scapularis in the east and upper Midwest, and Ixodes pacificus in the West (see Chapter 29). Major foci of infection exist in the upper Midwest, Northeast, mid-Atlantic, and parts of northern California (Figure 51-1).9 In some endemic areas, seroprevalence in dogs is nearly 90%,10 although seroprevalence in a study that used a C6 ELISA assay (see Diagnosis, later) was lower than this in endemic areas such as Connecticut and Massachusetts, around 20%.11 Infection is passed transstadially within the tick (i.e., from larva to nymph to adult), and not transovarially (from adult to egg). Reservoirs for the spirochete in the Northeast and the upper Midwest are Peromyscus leucopus, the white-footed mouse, which can harbor large numbers of the organism without overt signs of illness,12 as well as shrews and chipmunks.13 In the western United States, the western gray squirrel is the primary reservoir host.14 Lyme disease is less prevalent in the western United States because I. pacificus prefers to feed on the western fence lizard, a poor reservoir for the spirochete. Similarly, the low prevalence of Lyme disease in the southeastern United States, is because I. scapularis ticks feed primarily on lizards in this region.15 B. burgdorferi is primarily transmitted to humans by nymphal ticks, because they are extremely small and often enough go unnoticed. Adult ticks may be more important for transmission of infection to dogs.16 The nymphs of I. scapularis quest in the late spring and summer, when humans and dogs are often outdoors and become exposed. The peak questing times for I. scapularis adult ticks are in the spring and fall. Other vector-borne pathogens, such as Anaplasma phagocytophilum, may be co-transmitted and complicate the clinical picture. There is some evidence that co-infection with A. phagocytophilum can enhance the pathogenicity of B. burgdorferi.17 FIGURE 51-1 Approximate geographic distribution of Lyme borreliosis in humans (and dogs) in the United States. In continental Europe, Lyme disease is most prevalent in central and eastern Europe, especially Poland, Slovakia, and Slovenia, but also Germany, Switzerland, Austria, and southern Scandinavia (Figure 51-2). B. garinii was detected in a dog from the Czech republic with meningoencephalitis,18 and B. afzelii has been detected in dogs from Poland.19 Evidence of infection has also been found in dogs from the British Isles,20 where endemic areas include the Scottish Highlands, Ireland, Wales, the Lake District, the Yorkshire Moors, Exmoor, Wiltshire, Berkshire, and particularly the New Forest, the South Downs, and the Thetford Forest region. A huge variety of reservoir hosts appear to be involved in Europe. Small rodents such as squirrels appear to be an important reservoir host for B. afzelii, and birds such as blackbirds and song thrushes are important reservoir hosts for B. garinii.21 FIGURE 51-2 Countries and regions where Lyme borreliosis occurs in Europe. The size of the red dot correlates with the relative prevalence of the infection in those areas. The complete genome of B. burgdorferi has been sequenced. The organism expresses different outer-surface lipoproteins at different stages of infection, which allows it to adapt to dramatically different environments within the arthropod vector and the mammalian host. This property of the organism has been exploited for the purpose of diagnostic assay and vaccine development. Outer surface proteins of importance include OspA, OspC, and VlsE. Over the fall, winter, and spring, the spirochete remains dormant within the nymphal tick and expresses OspA, which allows it to adhere to the tick midgut. When the tick ingests mammalian blood in the late spring and summer, OspA expression is downregulated by the spirochete, and OspC expression is upregulated.22 The spirochete then moves to the tick hemolymph and salivary glands. OspC binds a tick salivary gland protein, which may help the organism evade the host immune response. OspC also binds to mammalian plasminogen and helps the spirochete to disseminate within the mammalian host.23 VlsE undergoes recombinational shuffling of its genetic code, which further allows the spirochete to evade the immune response.24 Finally, B. burgdorferi may undergo metamorphosis into a spherical shape when it encounters unfavorable conditions within the host, such as when it is exposed to antibiotics, nutrient deprivation, and changes in pH.25 This may also contribute to its ability to evade the immune system and resist antimicrobial drug treatment. Transmission is thought to require tick attachment for a minimum of 24 hours,26 but in some cases transmission may occur at earlier time points.27 The spirochete replicates at the site of tick attachment, then disseminates to multiple locations. Although it can be transiently found in blood, the organism primarily replicates and spreads through connective tissue (Figure 51-3). It binds proteins such as plasminogen, β3 integrins such as the platelet integrin αIIbβ3, glycosaminoglycans, fibronectin, laminin, and decorin (a collagen proteoglycan).28 After invasion, the organism can persist in dogs for over a year, through evasion of host immune responses.29 FIGURE 51-3 Borrelia burgdorferi in the metatarsal tendon sheath of a mouse with severe combined immunodeficiency. (Courtesy Dr. Stephen Barthold, Center for Comparative Medicine, University of California, Davis.) In contrast to human Lyme disease, dogs do not develop an early skin rash (erythema migrans). It has been estimated that only 10% of affected dogs show overt signs of illness. Initial signs in dogs occur 2 to 5 months after a tick bite and consist of variable fever, inappetence, thrombocytopenia, and lameness due to neutrophilic polyarthritis. In a study of experimentally infected dogs, the first joint to be affected was the joint closest to the site of the tick bite, which supports the notion that spirochetes reach the joints as a result of spread through connective tissue.29,30 Subsequently, other joints can be affected, and dogs may develop shifting lameness. It is not clear whether dogs develop other clinical manifestations seen in humans such as persistent, antibiotic-refractory Lyme arthritis; uveitis; carditis; or encephalopathy. Complete heart block was described in a seropositive dog from Connecticut that had pathologic changes at necropsy consistent with Lyme carditis.31 Lyme nephritis is a syndrome of membranoproliferative glomerulonephritis that has been recognized in dogs in association with exposure to B. burgdorferi. Rare reports of a similar disease in human patients exist.32 Golden and Labrador retriever breeds appear to be overrepresented,33 and it has been suggested that Shetland sheepdogs and Bernese mountain dogs might also be predisposed.34–36 Dogs with Lyme nephritis are younger than dogs with other glomerular diseases. In one study, more than 50% of dogs with Lyme nephritis were 5 years of age or younger.33 Many affected dogs are also thrombocytopenic, and some also have polyarthritis.33 The pathogenesis of Lyme nephritis is uncertain; immune-mediated mechanisms have been proposed.37 The DNA or antigen of the spirochete cannot be consistently detected within renal biopsies.37,38 Subendothelial deposits of IgM, IgG, and C3 can be detected within the glomeruli.33 Clinical signs result from proteinuria and renal failure and include inappetence, lethargy, weight loss, vomiting, and polyuria and polydipsia. Diagnosis of Lyme disease is notoriously difficult. The overwhelming majority of infected dogs experience subclinical infection, after which positive antibody titers may persist for months or years. Because only a small percentage (e.g., <10%) of infections result in disease, it is important to distinguish between diagnosis of infection or previous exposure to the spirochete and diagnosis of Lyme disease. Dogs with positive antibody titers may develop unrelated illnesses, which results in diagnostic confusion. As in human patients, the diagnosis must be established based on characteristic clinical signs, a history of exposure in an endemic area, and a positive antibody response to B. burgdorferi.39 A history of tick exposure may not always be present. The urinalysis in dogs with Lyme nephritis generally reveals isosthenuria and proteinuria. Pyuria and microscopic hematuria may also be present. Urine protein to creatinine ratios are often greater than 5 and may be above 15 in some dogs. Dogs with Lyme nephritis may have low antithrombin activities as a result of glomerular antithrombin loss, which may lead to hypercoagulability syndromes. Sometimes, abnormalities such as a shortened PT, prolonged APTT, and increased fibrinogen and D-dimer concentrations are present.40 Dogs with Lyme arthritis may have normal synovial fluid cytology,30 or there may be markedly increased numbers of nondegenerate neutrophils within the synovial fluid of distal joints (>5000 and frequently >10,000 cells/µL). Because not all joints may be affected, synovial fluid should be collected from at least three and preferably four peripheral joints. Specific diagnostic assays available for Lyme borreliosis in dogs are described in Table 51-3. Currently, one of the most widely used serodiagnostic tests for canine Lyme disease is based on a C6 ELISA, which detects antibodies against a portion of the VlsE lipoprotein. The advantages of the C6 ELISA assay are that (1) it detects IgG antibodies 3 to 5 weeks after the time of infection, so by the time dogs develop clinical signs they are virtually always seropositive,41 and (2) it is negative in dogs that have been vaccinated for Lyme disease, because the antigen is not expressed by organisms used in Lyme vaccines. The C6 ELISA is available as an in-practice lateral-flow assay, in combination with serodiagnostic spots for Ehrlichia canis/Ehrlichia ewingii antibody, Anaplasma spp. antibody, and Dirofilaria immitis antigen (SNAP 4Dx Plus, IDEXX Laboratories, ME) and as a quantitative ELISA (Quant C6), which is performed at IDEXX central veterinary diagnostic laboratories. There is no correlation between the magnitude of the C6 ELISA titer and disease severity. In a study from Europe, positive test results did not correlate with disease.36 Another rapid immunochromatographic ELISA assay has recently become available in the United States (Abaxis VetScan Canine Lyme Rapid Test) that detects antibodies to a different portion of the VlsE lipoprotein, OspC and p41; these are combined on a single line. Other serologic assays that are available include whole-cell ELISA or immunofluorescent antibody (IFA) assays and Western blotting (WB; see Chapter 2 for a description of these techniques). WB has traditionally been considered the gold standard assay. False positives using whole-cell ELISA and IFA have the potential to occur in patients with other spirochete infections, with other inflammatory disorders, and in vaccinated dogs. In human patients, a two-tiered approach is recommended for diagnosis.39 Serum from patients who test positive for IgM or IgG with ELISA is then subjected to WB, because WB has increased specificity. WB has been used to differentiate the response to immunization and natural infection in dogs (Figure 51-4).42 It has also been used to identify “dual status” dogs, that is, dogs that have been both immunized and naturally infected. WB is more time-consuming to perform than ELISA and IFA and experience is required to interpret it correctly.43 FIGURE 51-4 Western immunoblots of sera from dogs not infected with B. burgdorferi (Negative); immunized with recombinant OspA (vaccination rOspA); Immunized with a whole cell lysate vaccine (vaccination lysate); immunized and infected with B. burgdorferi (vaccination + infection); and infected only with B. burgdorferi (infection). The left strip shows all major signals available on the blot (control); the second strip from the left is stained with a monoclonal antibody against OspA (control OspA). (Courtesy R. Straubinger, Ludwig-Maximillians-Universitat, Munich, Germany. In: Greene CE: Infection Diseases of the Dog and Cat. 4th ed. St. Louis: Elsevier/Saunders; 2012.)
Lyme Borreliosis
Etiologic Agent and Epidemiology
Clinical Features
Diagnosis
Laboratory Abnormalities
Urinalysis
Coagulation Profile
Synovial Fluid Cytology
Microbiologic Tests
Serologic Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Lyme Borreliosis
Only gold members can continue reading. Log In or Register to continue