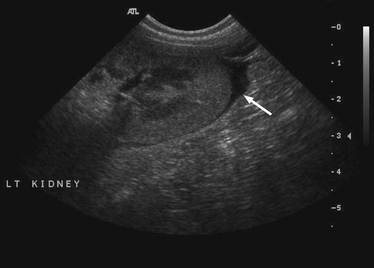
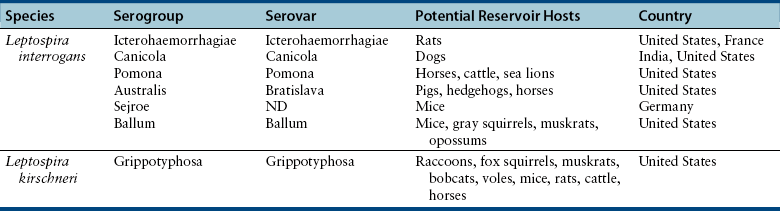
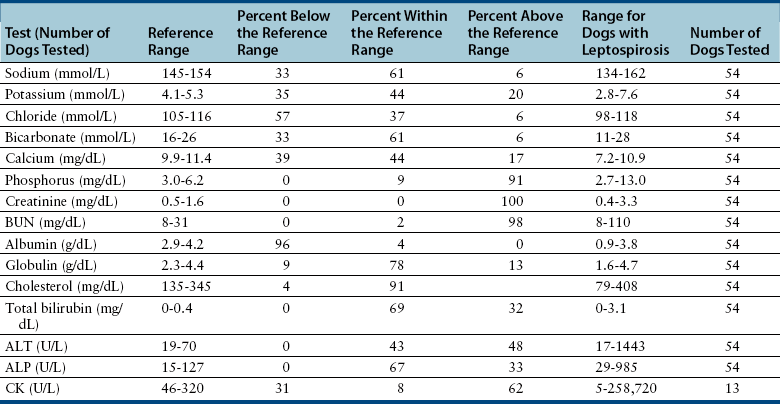
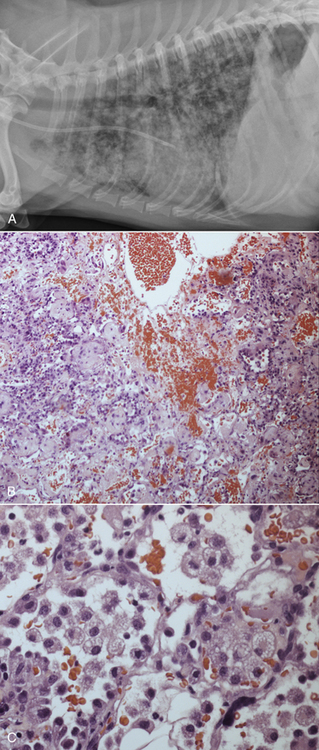
Chapter 50 Leptospirosis is a widespread zoonotic disease caused by systemic infection by pathogenic spirochetes of the genus Leptospira. Leptospira spp. are thin (0.1 µm in diameter), flexible, motile, spiral-shaped bacteria that have a hook-shaped end (leptos = “thin,” spira = “coiled”). A huge variety of different mammalian species may be infected by leptospires. These hosts can shed the organism in their urine and contaminate the environment. Infection of incidental hosts results in disease that varies in severity from a mild febrile illness to severe multisystemic disease. Both dogs and cats can develop leptospirosis, but cats appear to be relatively resistant to development of clinical illness. As a result, leptospirosis is uncommonly described in cats.2–5 Both pathogenic and saprophyte Leptospira species exist in nature. Saprophytic species, such as Leptospira biflexa, live in water and soil and do not infect animal hosts. Disease in dogs is primarily caused by the pathogenic species Leptospira interrogans and Leptospira kirschneri. Within the genus Leptospira, more than 250 different serovars of pathogenic leptospires are recognized, which are distinguished based on differences in their lipopolysaccharide (LPS) O antigens. The serovars are grouped into more than 20 antigenically related serogroups (Table 50-1). At least 10 serovars have been associated with disease in dogs worldwide. Each serovar is adapted to one or more wild or domestic animal reservoir host species (see Table 50-1). TABLE 50-1 Selected Serovars of Leptospira interrogans and Leptospira kirschneri that Infect Dogs ∗ ∗Isolated from dogs with naturally-occurring leptospirosis, or that induce disease in dogs after experimental inoculation. Recently, PCR-based DNA typing methods have been used to identify Leptospira strains on the basis of their genotype, instead of using the serovar classification method. Specific genotypes appear to associate more strongly with particular reservoir hosts and disease manifestations than has been found for serovars.6,7 Multiple different leptospiral serovars have been isolated from single outbreaks of human leptospirosis associated with specific clinical manifestations (such as pulmonary hemorrhage).8 Therefore, virulence factors other than outer surface antigens (which define serovars) may more important determinants of clinical manifestations of disease than the identity of the infecting serovar. Pathogenic Leptospira species do not replicate outside the host and are readily inactivated in the environment when exposed to excessive heat, ultraviolet irradiation, a variety of disinfectants, and freezing conditions. However, when conditions are optimal, pathogenic leptospires can survive in water and wet soil for weeks to months.9 Dogs that drink from, swim, or wade in environmental water sources can become infected; exposure to wildlife or farm animals and their urine is also a risk factor. In developing countries, dogs may be infected as a result of access to sewage.10 Although the “classic” signalment for a dog with leptospirosis is a large-breed, outdoor, intact male dog, dogs of any age, breed, and sex may be affected. For example, geriatric, small-breed dogs that live in urban or suburban areas and that contact rodents or rodent urine can develop the disease. Indeed, over the past decade, residence in urban areas has emerged as a risk factor for Leptospira infection in dogs in some parts of North America, possibly due to exposure to urban wildlife species.11 Cats may be infected after ingestion of infected prey, so outdoor exposure and hunting behavior may be risk factors for cats.2 Outbreaks of disease in dogs have been correlated with periods of high annual rainfall and may occur approximately 3 months after wet weather.12–14 The peak seasonal distribution in parts of North America where freezing winters occur is late fall,11,15,16 but in more temperate regions, peak seasonal distribution occurs after months of high rainfall (such as in the winter in northern California).13 Leptospirosis is most prevalent in parts of the world where there is higher annual rainfall and a warm climate, but the presence of infected reservoir hosts also influences the geographic distribution of leptospirosis. Within North America, dogs that live in or travel to Hawaii, northern California, Oregon, Washington, the upper Midwest and Midwest, parts of Texas, Colorado, and the northeastern and mid-Atlantic coastal regions may be more likely to develop infection.17 Leptospirosis also occurs in dogs in the southeastern United States,18 and the disease has been widely reported in dogs from Ontario in Canada.16 Numerous reports describe leptospirosis in dogs from across Europe.19 Major disease foci among people include the Caribbean, Central and South America, India, Southeast Asia, South America, and Oceania. Worldwide, the most common serovars thought to infect dogs before the introduction of canine Leptospira vaccines several decades ago were Icterohaemorrhagiae and Canicola.20 Since that time, widespread seroconversion to other serovars, especially Grippotyphosa and Pomona in North America, has been noted in sick dogs. Increased testing for these serovars may have contributed to this phenomenon, as well as increased contact between dogs and wildlife and farm animal reservoir hosts for these serovars.21 Serovars that currently cause disease in the dog population are poorly understood, because the results of serology do not accurately predict the infecting serovar,22 and culture and identification of leptospiral serovars is insensitive, requires significant technical expertise, and is not widely available (see Diagnosis, later).23 The incubation period for acute leptospirosis is approximately 7 days, but may be shorter or longer depending on the dose and strain virulence of the leptospires involved, and the host immune response.24 Dogs can also develop signs of chronic kidney disease months or more than a year after acute kidney injury. Early renal damage in this situation may be associated with transient or undetected clinical signs, which are subsequently followed by either clearance or persistence of infection and progression of renal damage. Longer incubation periods might occur in cats,2 but further study is required. Leptospirosis varies considerably in severity, which may contribute to underrecognition of the disease. Many dogs probably show no signs of illness or develop only a mild, transient febrile illness. Severe clinical signs and death result from renal failure and/or injury to the liver and lungs. Leptospirosis should be suspected in dogs that develop signs of acute febrile illness, kidney and hepatic injury, uveitis, or pulmonary hemorrhage; it should also be suspected in breeding dogs with abortion, stillbirths, or neonatal deaths.20 Clinical signs in affected cats resemble those in dogs, although some cats have lacked evidence of hepatic injury.2 Fever occurs only at the onset of illness and is often accompanied by shivering, reluctance to move, and generalized muscle tenderness that may result from myositis. This may be followed by signs of lethargy, polyuria and polydipsia, inappetence, vomiting, and diarrhea.24–27 Gastrointestinal signs in some dogs may result from pancreatitis and enteritis, in addition to acute renal and liver damage. Polyuria and polydipsia may result from a decreased responsiveness of the inner medullary connecting ducts to antidiuretic hormone (acquired nephrogenic diabetes insipidus). However, some dogs show decreased thirst and urination. Liver involvement leads to icterus in some dogs (Figure 50-1). Components of leptospiral endotoxin such as oleic and linoleic acid are thought to interfere with hepatic transport pumps, which leads to functional hepatic impairment.28 Hepatocyte apoptosis and injury to hepatocyte membranes is also suspected to occur; leptospirosis in humans and guinea pig models is associated with loss of the cellular adhesion molecule E-cadherin on hepatocytes.20 FIGURE 50-1 Uveitis in an Australian shepherd with leptospirosis with leakage of serum bilirubin into the aqueous humor. Less commonly, uveitis, myocardial damage, or reproductive complications occur. Leptospiral DNA can be detected in the aqueous humor of human patients with leptospiral uveitis, and the development of uveitis may be delayed up to 18 months after the onset of acute illness.29 ECG alterations can occur in dogs that suggest myocardial damage.30 Abortion can occur during pregnancy.31 Stillbirth and neonatal deaths have also been described. Bleeding tendencies in dogs with leptospirosis may be manifested as petechial hemorrhages, hematuria, hematemesis, epistaxis, hematochezia, or melena.25,30,32 Although the precise mechanisms are unknown, bleeding tendencies may reflect the presence of endothelial damage and disordered coagulation. In human patients, thrombocytopenia (≤100,000 platelets/µL) and prolonged PT have been associated with bleeding.33,34 Tachypnea may be noted as a result of severe pulmonary hemorrhage syndrome (SPHS).35,36 SPHS is recognized commonly in humans with severe leptospirosis, is not associated with significant lung colonization by the spirochete, and seems to have an immune-mediated pathogenesis.37 The prevalence of SPHS in dogs appears to be particularly high in continental Europe. For example, pulmonary complications occurred in more than two thirds of dogs with leptospirosis in Berlin.36 Vasculitis can also contribute to the development of peripheral edema and mild pleural and peritoneal effusions. In human patients, aseptic meningitis is a common complication of leptospirosis and results in signs of severe headache, delirium, and less commonly seizures and altered sensory perception.38 Whether meningitis occurs in dogs is unknown. The extent to which leptospires can cause chronic hepatic injury in dogs is also unknown. In one report from France, leptospires were detected in bile canaliculi of young dogs with chronic hepatitis using immunohistochemistry and electron microscopy. These dogs showed signs of poor growth, weight loss, and ascites.39,40 Attempts to detect Leptospira DNA in liver tissue from dogs with chronic active hepatitis have been unrewarding. The hemogram in dogs with leptospirosis may reveal neutrophilia, sometimes with bandemia; nonregenerative anemia; lymphopenia; and/or thrombocytopenia (Table 50-2). Thrombocytopenia occurs in up to 53% of dogs.25,26,30,41 The mechanism is unclear; disseminated intravascular coagulation (DIC) or immune-mediated mechanisms may be involved. Findings on the serum chemistry panel vary depending on leptospiral strains that circulate in a particular geographic region (Table 50-3). In all geographic locations, a combination of azotemia and elevated liver enzyme activities, especially when thrombocytopenia is present, should raise suspicion for leptospirosis. Azotemia is generally present in more than 80% to 90% of dogs,25,26,30,42 although in one European study, increased serum creatinine concentration was present in only 57% of affected dogs.41 Uncommonly, dogs exhibit increased liver enzyme activities in the absence of azotemia. Signs of hepatic dysfunction are associated with minimal histologic damage to the liver and include variable, and generally mild to moderate, increases in serum ALT, AST, and ALP activities and total bilirubin concentration. TABLE 50-3 Serum Biochemistry Findings at Admission in Dogs with Leptospirosis from Northern California∗ ∗Diagnosis based on results of acute and convalescent phase serology together with consistent clinical signs. Other findings on the serum chemistry panel include hypoalbuminemia and a variety of electrolyte abnormalities. Hyponatremia, hypochloremia, or hyperphosphatemia occur in most dogs. Decreased expression of sodium transporters by proximal convoluted tubule cells contributes to impaired sodium resorption, increased distal sodium delivery, and potassium wasting.43 As a result, dogs with acute oliguric renal failure may have paradoxical hypokalemia. Moderately and occasionally markedly increased serum CK activity is present in some dogs due to myositis. Increased serum troponin concentrations have been found in some dogs with leptospirosis, which suggests myocardial damage.30 Increased serum pancreatic lipase immunoreactivity may occur, probably as a result of pancreatitis. Findings on urinalysis in dogs with leptospirosis include isosthenuria, occasionally hyposthenuria, and variable glucosuria, proteinuria, pyuria, cylindruria, and bilirubinuria.20,26,30,42 Urine protein to creatinine (UPC) ratios may be increased. Glucosuria was present in 77% of 44 dogs with leptospirosis at the author’s institution, and in 21 dogs that had UPC ratios measured, the ratios ranged from 0.4 to 11.6 (median, 3, reference range less than 0.5). Most proteins in the urine are of low molecular weight, which supports a tubular rather than glomerular origin of these proteins.30,44 Leptospires are not visible in urine sediment by routine light microscopy. Aside from thrombocytopenia, analysis of hemostatic function in dogs with leptospirosis shows variable increases in fibrinogen, D-dimer, and fibrinogen degradation product concentrations. Antithrombin activity may be decreased. Prolongations in PT and PTT have been present in up to 15% of tested dogs.20,26,30 PT prolongations are associated with mortality in human patients.34 In some dogs, a shortened PT is present,42 possibly because of DIC. In human patients, leptospirosis has been associated with an imbalance between coagulation and fibrinolysis.34 Plain thoracic radiography in dogs with leptospirosis may show no abnormalities, or mild to severe, diffuse interstitial pulmonary patterns may be present. Thoracic radiographs from dogs with SPHS can show severe nodular to diffuse interstitial and alveolar patterns (Figure 50-2). Uncommonly, mild pleural effusion is present. FIGURE 50-2 Clinical images from a 4-year-old male neutered Havanese terrier with severe leptospiral pulmonary hemorrhage syndrome and anuric renal failure. A, Right lateral thoracic radiograph. A severe, diffuse alveolar pattern is present. Mechanical ventilation was needed and ultimately euthanasia was performed because of respiratory failure. Histopathology of a lung biopsy showed severe pulmonary hemorrhage (B) and marked alveolar histiocytosis (C). Findings on abdominal sonography include mild renomegaly, increased renal cortical echogenicity, mild pyelectasia, perirenal fluid accumulation or mild ascites, and a medullary band of increased echogenicity within the kidneys (Figure 50-3).45 None of these signs are specific for leptospirosis. Ultrasonographic changes suggestive of pancreatitis are occasionally present, such as enlargement and hypoechogenicity of the pancreas. Thickening of the gastric, and less commonly small intestinal wall may also be present. Mild to moderate hepatomegaly and splenomegaly with mottling of the splenic echotexture may be detected, with or without evidence of mild abdominal lymphadenomegaly. Diagnostic assays available for leptospirosis in dogs are described in Table 50-4. Most often, diagnosis is based on acute and convalescent phase serology. Only certain reference laboratories have expertise in culture of leptospires and the ability to identify leptospires to the serovar level, which is performed using a cross agglutinin absorption test. Genetic typing methods such as pulsed field gel electrophoresis can also be performed.23 Despite the difficulties associated with culture and identification of leptospires, a proper understanding of the epidemiology of leptospirosis depends on the use of these methods.
Leptospirosis
Etiologic Agent and Epidemiology
Clinical Features

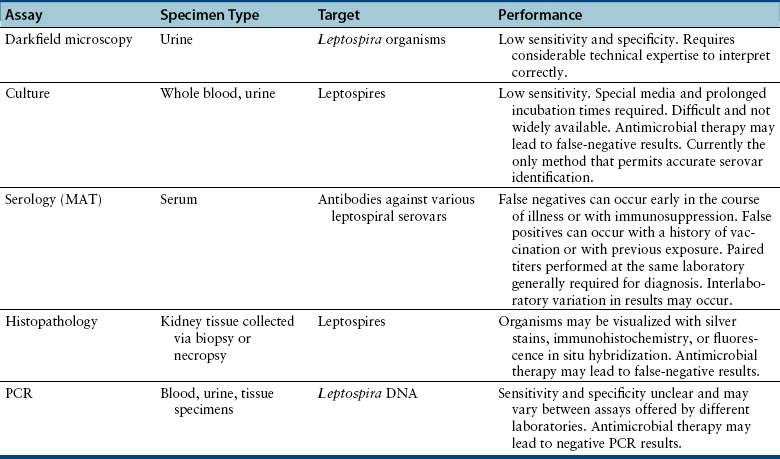
Diagnosis
Complete Blood Count
Serum Biochemical Tests
Urinalysis
Coagulation Function
Diagnostic Imaging
Sonographic Findings
Microbiologic Tests
Culture
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Leptospirosis
Only gold members can continue reading. Log In or Register to continue