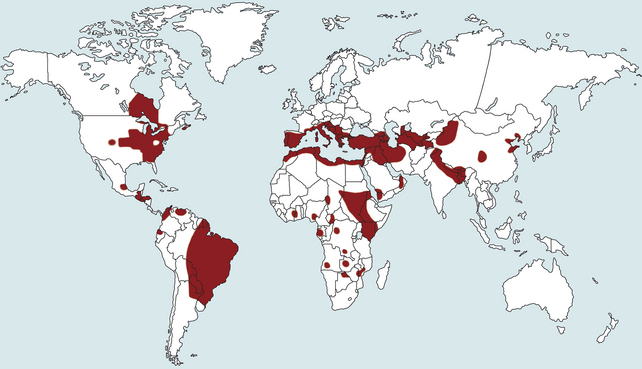
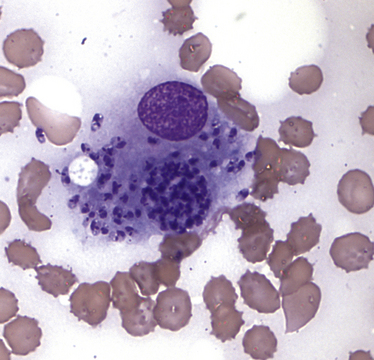
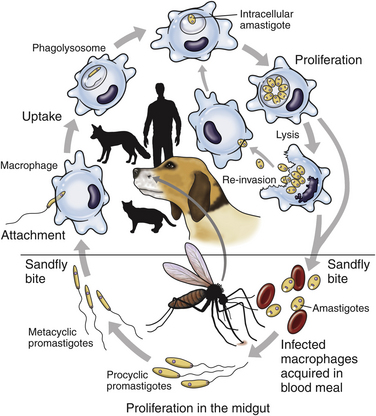
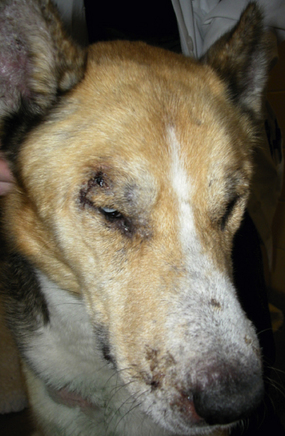
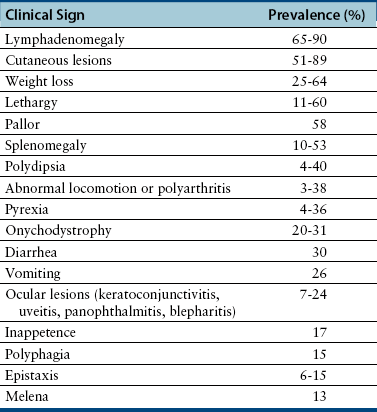
Chapter 74 The leishmaniases are a group of zoonotic vector-borne diseases caused by various species of the protozoa Leishmania, which are primarily transmitted by sandflies. Around 30 species of Leishmania exist worldwide, and at least 20 species cause disease in humans, although the taxonomy of Leishmania spp. has been highly debated.2,3 There are two subgenera of Leishmania, Leishmania and Vianna, which develop in the foregut or the hindgut/midgut of the sandfly vector, respectively. Human and animal disease due to Leishmania spp. is distributed across five continents (Africa, Asia, Europe, and the Americas) and nearly 100 countries (Figure 74-1). Disease syndromes in humans are divided into cutaneous leishmaniasis (CL), mucosal leishmaniasis (ML), and visceral leishmaniasis (VL). Disease syndromes in dogs are divided into visceral leishmaniosis and American tegumentary leishmaniosis, a localized cutaneous form that occurs in parts of South America. The term ‘leishmaniosis’ as opposed to ‘leishmaniasis’ is preferred to distinguish the disease in animals from that in humans. The life cycle of Leishmania alternates between two major forms: the promastigote and the amastigote (Figure 74-2). The promastigote resides in the gut of the sandfly vector and is an elongated, flagellated form. Promastigotes are inoculated into tissues when the sandfly feeds, where they are phagocytized by macrophages in the dermis and transform into intracellular, nonmotile, ovoid, or round amastigotes. The amastigotes survive and replicate within macrophage phagolysosomes, and eventually the infected macrophages rupture. Released amastigotes then infect other macrophages that have been attracted to the site of infection. If the infected host fails to control the infection, amastigotes disseminate via regional lymphatics and the blood to infect the entire reticuloendothelial system. Intracellular amastigotes that are ingested by a female sandfly during feeding convert back into promastigotes over a period of about 1 week, and replication of promastigotes within the sandfly completes the life cycle. Sandflies breed in cracks in the walls of dwellings, rubble, and in rodent burrows, feed primarily at night, and fly a maximum of 2.5 km from their breeding sites. As a result, the prevalence of infection in dogs can vary considerably between adjacent regions. Lesser modes of transmission of Leishmania spp. include vertical transmission from bitches to puppies, venereal transmission, and blood transfusion.4–7 These modes of transmission become more obvious in regions where suitable sandfly vectors do not exist. Aggressive interactions between dogs also have the potential to transmit infection, but this route has not been proven. FIGURE 74-2 Life cycle of Leishmania infantum. Dogs, cats, and humans become infected when they are bitten by a sandfly. Promastigotes are inoculated into the dermis and penetrate macrophages. Within the macrophage, they transform into amastigotes and replicate within a phagolysosome. The macrophage ruptures and new macrophages are infected. If the host fails to control the infection in the skin, amastigotes disseminate via regional lymphatics and the blood to infect the entire reticuloendothelial system. Amastigotes that are ingested by a female sandfly during feeding convert back into promastigotes, and replication of promastigotes within the sandfly completes the life cycle. Canine visceral leishmaniosis in dogs and cats is caused by Leishmania infantum (also referred to as Leishmania chagasi in Central and South America). L. infantum is endemic in the Mediterranean basin, the Middle East, and Asia and may have been introduced to Latin America by early explorers and their domestic dogs.8 Because leishmaniosis has a long incubation period and prolonged disease course, travel-related leishmaniosis in dogs and cats is diagnosed sporadically in regions where sandfly transmission does not occur, such as parts of Scandinavia, Switzerland, Japan, the United Kingdom, and the western United States (most often in dogs imported from southern Europe).9–13 In highly endemic regions of the Mediterranean and Brazil, infection rates among dogs vary widely but may reach 80%.14–17 At least 2.5 million dogs are infected in southwestern Europe alone.14 The disease may be spreading northward in Europe, and has recently appeared in nontraveled dogs in countries such as Germany and Hungary.5,18,19 Spread of Leishmania spp. into new areas can occur when humans bring infected dogs into regions where competent insect vectors exist, or when sandfly populations expand as a result of factors such as climate change. Risk factors for infection in dogs include age at least 2 years, prolonged exposure to the outdoors, lack of topical insecticide use, and, in some studies, a short haircoat.20–23 Some authors have described a bimodal age distribution that peaks at 3 years and at greater than 8 years of age.24 Sex is not an important risk factor for infection. Factors that promote proliferation of sandfly populations, such as poor sanitation and limited garbage collection, as well as the occurrence of stray dogs around homes, also put dogs at risk of infection.25 In Europe, German shepherd dogs, boxers, Rottweilers, cocker spaniels, and Dobermans may be at increased risk for leishmaniosis; the Ibizan hound in Spain appears to be resistant to clinical disease.24,26–29 Concurrent diseases are common in dogs with leishmaniosis in endemic regions and include transmissible venereal tumor,30 demodicosis,31 sarcoptic mange,32 and a variety of other vector-borne diseases such as canine monocytic ehrlichiosis, babesiosis, hepatozoonosis, and dirofilariasis.32–36 Concurrent lymphoma has also been described.32 Uncommonly, dogs worldwide are infected by other species of Leishmania, which include Leishmania donovani, Leishmania tropica, Leishmania braziliensis, Leishmania peruviana, Leishmania mexicana, Leishmania panamensis, Leishmania guyanensis, Leishmania amazonensis, and other as-yet-uncharacterized species.37–45 L. braziliensis, L. guyanensis, and L. panamensis have been isolated from dogs with American tegumentary leishmaniosis. Outbreaks of American tegumentary leishmaniosis have occurred in humans and dogs.43 Most sporadic cases of visceral leishmaniosis in dogs in North America are diagnosed in dogs with a travel history to other continents. Infected dogs continue to be introduced every year as a result of travel from endemic regions in Europe and Latin America. In addition, over the past 30 years, visceral disease has been described in dogs in the United States that have had no apparent travel history. These have included a basenji dog from a kennel in Texas and dogs of foxhound breed from Ohio, Oklahoma, and Colorado.46–49 Involvement of multiple dogs in a foxhound kennel was reported in Oklahoma,47 and outbreaks in foxhounds have also been described in New York, Alabama, and Michigan.50 Spread to other dogs has occurred as a result of transplacental transmission and, in rare cases, transfusion of infected blood.4,6 A competent arthropod vector for Leishmania spp. has not yet been identified in the United States, and infection has not been detected in surveys of non-foxhound dogs in the United States.51 The outcome of Leishmania spp. infection is variable. Some dogs completely eliminate the infection, and a small percentage of dogs develop severe, life-threatening disease. Some dogs remain persistently but subclinically infected, with the possibility of reactivation later in life with immunosuppression; these dogs also act as reservoir hosts and can infect sandflies.52 In general, dogs that mount strong T-cell–mediated immune responses eliminate the infection, whereas those that mount weak cell-mediated immune responses and marked, nonprotective humoral (IgG) immune responses develop clinical disease.53 Host genetic factors are important in determining progression to clinical disease, and several candidate genes have been identified in dogs that may influence susceptibility.28,54–56 When clinical disease appears, it occurs after a long incubation period (up to 7 years) and varies from mild papular or exfoliative dermatitis to severe disseminated disease due to massive proliferation of histiocytes, B lymphocytes, and plasma cells in reticuloendothelial tissues and immune-mediated consequences of chronic, persistent infection. Cutaneous lesions most commonly manifest as alopecia, scaling, and/or ulceration but can be nodular or papular (Figure 74-3). They are often noticed before signs of systemic infection develop, despite the fact that they occur as a consequence of parasite dissemination. Many dogs also develop onychogryphosis (abnormally long or brittle claws). FIGURE 74-3 Periocular alopecia and crusting in an 8-year-old male neutered husky mix with visceral leishmaniosis. The dog was evaluated in northern California but had a travel history to Spain. Lesions are also present on the muzzle and the pinnae. Systemic signs of visceral leishmaniosis include fever, weight loss, muscle atrophy, inappetence, and lethargy; oral ulceration; progressive splenomegaly and lymphadenomegaly; mucosal pallor due to anemia; and rarely, hepatomegaly (Table 74-1). Development of autoantibodies and circulating immune complexes is thought to lead to immune-mediated thrombocytopenia and/or thrombocytopathia and signs such as epistaxis or melena, lameness and joint swelling due to immune-mediated polyarthritis, myositis, uveitis, vasculitis, and glomerulonephritis.57–62 Positive antinuclear antibody tests are found in up to 50% of dogs tested.32,61,63 Many dogs also have positive Coombs’ tests.32,63 TABLE 74-1 Clinical Signs in Dogs with Visceral Leishmaniosis Data from Ciaramella P, Oliva G, Luna RD, et al. A retrospective clinical study of canine leishmaniasis in 150 dogs naturally infected by Leishmania infantum. Vet Rec 1997;141:539-543; Slappendel RJ. Canine leishmaniasis. A review based on 95 cases in The Netherlands. Vet Q 1988;10:1-16; Koutinas AF, Carlotti DN, Koutinas C, et al. Claw histopathology and parasitic load in natural cases of canine leishmaniosis associated with Leishmania infantum. Vet Dermatol 2010;21:572-577. Glomerular injury ultimately culminates in nephrotic syndrome and/or failure of tubular function for many dogs, with polyuria and polydipsia, vomiting, diarrhea, and dehydration. Renal failure may occur in the absence of other lesions.32 Anemia may be due to a combination of factors that include blood loss, inflammation, renal failure, immune-mediated destruction, and marrow aplasia or hypoplasia. In addition to uveitis, other ocular lesions include blepharitis, conjunctivitis, panophthalmitis, and keratoconjunctivitis. Keratoconjunctivitis results from direct infection of the lacrimal gland by Leishmania.64 Atypical presentations of canine visceral leishmaniosis have also been reported. Almost any organ can be affected, with reports of Leishmania prostatitis and infertility65; nodular lesions of the tongue and other mucosal surfaces66,67; pancreatitis68; gastrointestinal tract, myocardial, or pulmonary involvement69–71; osteomyelitis72,73; meningitis74; and granuloma formation within the spinal cord.75 American tegumentary leishmaniosis in dogs is characterized by development of a nodular skin lesion at the site of a sandfly bite that enlarges and eventually may ulcerate. Lesions most often occur on regions of the body that have less hair, such as the nose, perineal region, scrotum, and pinnae.43,76 Physical examination findings in dogs with visceral disease include lethargy, muscle atrophy or cachexia, variable and typically low-grade fever, focal or generalized peripheral lymphadenomegaly, and hepatosplenomegaly on abdominal palpation (Figure 74-4). Lymphadenomegaly can be severe and be suggestive of lymphoma. Although not always detected, skin lesions consist of scaling and crusting, hyperkeratosis, alopecia, erythema, and cutaneous ulceration and may be nonpruritic or pruritic. They most often occur on the limbs, pinnae, muzzle, and periocular regions. Mucocutaneous lesions can also occur. Ocular involvement may be associated with mucopurulent ocular discharge, blepharitis, and anterior uveitis. Onychogryphosis may be present. Lameness or reluctance to move may be associated with joint swelling, crepitus, and pain.72 Epistaxis may also be noted. Diagnosis of leishmaniosis may be straightforward, such as when organisms are seen cytologically in impression smears of typical skin lesions, or it may require assessment of the results of a combination of diagnostic assays in light of clinicopathologic findings (Table 74-2). Specific diagnostic assays include cytologic or histopathologic examination of affected tissues, serology, culture of the organism, or the results of PCR assays on affected tissues or blood. In endemic areas, diagnosis may be challenging (1) because dogs may be infected with the parasite for long periods of time in the absence of clinical signs, so the significance of positive test results may be unclear, and (2) because co-infections with other vector-borne pathogens may complicate the clinical picture and cause signs that resemble those of leishmaniosis. The most common CBC abnormality in dogs with leishmaniosis is a mild to moderate, normocytic, normochromic, nonregenerative anemia. Mild thrombocytopenia is present in up to 50% of affected dogs.32,63,77 Total leukocyte, neutrophil, lymphocyte, monocyte, and eosinophil counts may be decreased, within the reference range, or increased. Pancytopenia may be present. Although most dogs are lymphopenic, some have moderate to severe lymphocytosis.77 Changes on serum biochemistry analysis in dogs with leishmaniosis include hyperglobulinemia, hypoalbuminemia, and mild to moderate azotemia.32,63,77 Hyperglobulinemia and hypoalbuminemia are present in more than 75% of affected dogs. Serum globulin concentration ranges from mild to severe (up to 8 g/dL) and reflects a polyclonal gammopathy; monoclonal gammopathy is rare.78 Azotemia has been reported in 16% to 38% of dogs. Some studies have shown mild to moderate increases in liver enzyme activities. Cytologic examination of synovial fluid from dogs with Leishmania polyarthritis reveals increased numbers of neutrophils and/or lymphocytes.72 In some cases, Leishmania amastigotes are visible within macrophages in the synovial fluid. Bone marrow cytologic alterations described in canine leishmaniosis include erythrophagocytosis, erythroid hypoplasia and dysplasia, eosinophilic hypoplasia, granulocytic hyperplasia, and increased numbers of lymphocytes and plasma cells.79,80 Amastigotes may also be visible. Abdominal radiography or ultrasonography in dogs or cats with visceral leishmaniosis can reveal abdominal lymphadenomegaly, splenomegaly, and hepatomegaly. Radiographic lesions of long bones in dogs with bone involvement consist of soft periosteal proliferation, increased or decreased intramedullary opacity, and/or cortical and medullary destruction.63,72 Lesions tend to be bilateral and symmetrical. Joint radiographs in dogs with signs of arthritis may reveal erosive changes, or arthritis may be non-erosive. Identification of Leishmania spp. amastigotes in lesions with cytology is diagnostic for leishmaniosis (Figure 74-5). Organism densities are particularly high in skin lesions, bone marrow, and the spleen, but specimens collected from other tissues, such as lymph node and hepatic aspirates, may also contain organisms. Organisms are not found in some lesions, and inexperienced microscopists may fail to identify organisms when present or confuse them with other parasites, such as Trypanosoma, Histoplasma, or Sporothrix schenckii. Rarely, organisms are seen in circulating white blood cells.81 Serologic assays based on immunofluorescent antibody (IFA), ELISA, Western immunoblot, and agglutination techniques have all been used for serodiagnosis of canine leishmaniosis, or for screening apparently healthy dogs in endemic areas for evidence of infection. IFA is commonly used by veterinary practitioners in Europe82 and has a diagnostic sensitivity that ranges from 90% to 100% and a specificity of 80% to 100% in dogs that have not received a Leishmania vaccine. Dogs with Trypanosoma spp. infections may have false-positive test results due to serologic cross-reactivity between Trypanosoma and Leishmania antigens. ELISA assays based on recombinant antigens often have higher specificity and are technically less difficult to perform than IFA.42,83 A rapid lateral flow assay is available commercially (SNAP Canine Leishmania Antibody Test Kit, IDEXX Laboratories) that had an overall sensitivity of 95% and a specificity of 91% in one study of 400 dogs from Brazil.83 Other immunochromatographic assays are available that vary in their sensitivity. Negative serologic test results may reflect delayed seroconversion in some animals, which can take several months to occur. However, many dogs with clinical signs have already seroconverted because of the long incubation period. Importantly, in endemic areas, positive serologic test results do not imply that disease is due to Leishmania spp. infection, because subclinical infections are widespread. Thus, for diagnosis of clinical leishmaniosis, results must be interpreted in conjunction with clinical findings and cytologic and histopathologic evaluation of affected tissues. Because seroconversion occurs in dogs that have received the Leishmania vaccine, current serologic assays cannot be used to efficiently differentiate between infected and vaccinated animals.84 Several different conventional and real-time PCR assays have been developed and evaluated for detection of Leishmania spp. in dogs and cats. Real-time PCR assays that detect Leishmania spp. are now offered on a commercial basis by some veterinary diagnostic laboratories worldwide. Suitable specimens for testing include splenic, hepatic, lymph node, or bone marrow aspirates; whole blood; skin biopsies; conjunctival swabs; and even oral swabs. Conjunctival swabs; aspirates of spleen, bone marrow, or lymph nodes; and skin biopsies are most likely to yield positive results.85,86 Positive PCR assay results in endemic areas do not confirm that disease is caused by Leishmania spp., because dogs without clinical signs can test positive.87 However, in one study, clinical disease subsequently appeared in 71% of ELISA-positive dogs that lacked clinical signs but had medium to high parasite loads (more than 10 parasites/mL of blood) as determined with a quantitative PCR assay.86 PCR assays may also be useful in order to monitor the efficacy of treatment.
Leishmaniosis
Canine Leishmaniosis
Canine Leishmaniosis in North America
Clinical Features
American Tegumentary Leishmaniosis
Physical Examination Findings
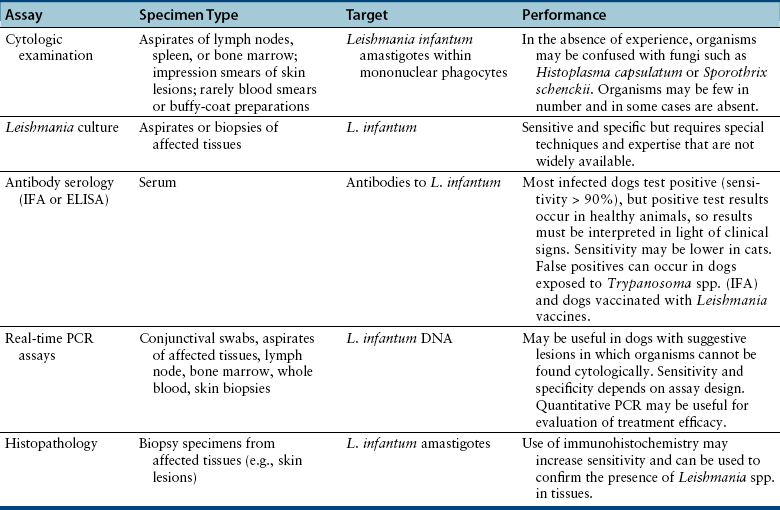
Diagnosis
Laboratory Abnormalities
Serum Biochemical Tests
Synovial Fluid Examination
Bone Marrow Examination
Diagnostic Imaging
Microbiologic Tests
Serologic Diagnosis
Molecular Diagnosis Using the Polymerase Chain Reaction
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine