Lactation and Neonatal Care
An epidemiologic study in the United Kingdom reported that 4% to 11% of the deaths among llamas and 17% to 33% of deaths in alpacas occur during the first 6 months of life. A high proportion of these deaths occur within the first week of life.1 In South America, cria mortality rates before weaning may reach 50%, mostly caused by Clostridium perfringens (types A and C).2 As in other domestic animal species, most neonatal deaths are associated with events occurring during parturition and the immediate postpartum period such as dystocia, poor mothering ability, or exposure. Poor mothering ability includes not only behavior problems such as rejection but mostly poor milk production. Neonatal care and early recognition and treatment of neonatal ailments are of the utmost importance to reduce these losses.
Prenatal Care
Care of the neonate begins during the long gestation as strength and viability of the newborn is greatly influenced by health of the dam.3 More than 80% of fetal growth occurs in the last trimester of pregnancy, and the normal rate and growth pattern of the fetus may be altered by numerous factors, including abnormal hormonal environment, nutrition, genetics, and infectious conditions.3,4 These factors may cause chronic or acute disruption of development of the fetus, resulting in a stillborn or dysmature neonate without any obvious clinical problems in the dam. Taking these factors into consideration, prenatal care of the dam with proper nutrition, vaccinations, and knowledge of any concurrent herd health problems is important to enhance survival of the newborn. All these aspects have been discussed in detail in other chapters in this text.
Postnatal Care
Even with an optimal uterine environment, the newborn is subject to severe stresses and some degree of oxygen deprivation during parturition. During parturition, it is possible that the neonate may be affected by a damaging degree of anoxia. Several mechanisms help the neonate to adapt to extrauterine life. The first is an increase in fetal cortisol concentration, which triggers parturition and allows adequate levels of surfactant to be produced by type II alveolar pneumocytes. In addition to elevated cortisol concentration, a catecholamine surge occurs. A negative side effect of this physiologic feature is that it may allow potential problems to be masked and the newborn to appear normal immediately after parturition, even though it may have substantial physiologic impairment.5
Early diagnosis and aggressive treatment of neonatal disease, particularly infections, results in greater positive outcome.6 The clinical signs are often nonspecific and vague, which results in a neonate that is slow to adapt to extrauterine life or that dies suddenly in the first few days of life. If an intrauterine infection exists, the fetus may be born alive or may die in utero. Intrauterine infections of bacterial origin in the newborn camelid are recognized more commonly compared with viral infections. Infections acquired in utero rather than in the postpartum period should be suspected if the newborn has elevated plasma fibrinogen in the first 12 to 24 hours of life, the placenta appears abnormal, or the dam exhibits vaginal discharge during parturition.6,7
Normal Behavior of the Newborn Cria
The newborn cria should be evaluated within the first few hours of life for any abnormalities of development or maladjustment to extrauterine life. Physical and behavioral parameters of the normal newborn camelid are presented in Table 25-1. Assessment of the newborn cria includes evaluation of the epidermal membrane, respiration, heart function, and presence of obvious congenital abnormalities. The remaining epidermal membrane should be removed with clean towels. It is normally translucent and may become yellow or brownish because of meconium staining in cases of fetal stress (fecal passage caused by dystocia). Ideally, the placenta should be weighed and examined thoroughly for completeness and signs of inflammation or infection, as described in the chapter on postpartum disorders in this text (Chapter 26, Postpartum Disorders). Clients should be instructed to keep the placenta refrigerated for any subsequent diagnostic examination, as needed.
TABLE 25-1
Biologic Parameters of Normal Healthy Newborn Camelids
| Parameter | Reference Range? |
| Birth weight (kilograms) | 5 to 11 alpacas (Vicugna pacos) 9 to 18 llamas (Lama glama) |
| Temperature (°C) | 37.7 to 38.9 |
| Pulse (beats per minute [beats/min]) | 60 to 100 |
| Respiration (beats/min) | 10 to 30 |
| Time to standing (minutes) | 30 (10 to 120) |
| Time to nursing (minutes) | 45 (20 to 180) |
| Nursing frequency | Several sessions per hour lasting 1 to 3 minutes |
| Meconium passage (hours)* | <18 hours |
| Urination (hours) | <18 hours |
| Daily gain Kg (first 3 months of life)** | 0.2 to 0.4 (alpaca), 0.4 to 0.8 (llama) |
*Meconium passage should occur about 8 hours after a normal feeding.
**Most neonates will register a slight drop in weight in the first 24 hours.
The cria should be weighed daily to monitor weight gain accurately. Regular weighing of the cria (daily for the first 2 weeks and once every other week thereafter) is warranted to determine the adequacy of milk production and intake. Birth weights vary significantly from one farm to another and reflect most likely the feeding management on that farm as well as genetic influence (Table 25-2). A tendency toward higher birth weight than originally described has been observed in many alpaca farms in North America. In alpacas in South American conditions, birth weights have tended to increase with increasing age of the dam, and low birth weights have been associated with an increased risk of neonatal death.8 Birth weights tend to decrease after 11 years.8 No studies have been performed on the effect of the sire on birth weight in these species. It is not uncommon for crias to lose some weight in the first 24 hours (120 to 250 grams [g] in alpacas and 250 to 500 g in llamas). In alpacas, the average daily weight gain in crias is 195 g under grazing conditions in South America.3
TABLE 25-2
Variation of Birth Weight in Normal Alpaca Crias from a Herd in the United States
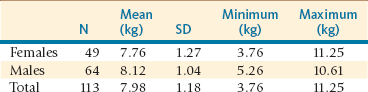
kg, Kilogram; N, number; SD, standard deviation.
From Tibary A, et al: Neonatal care and neonatal emergencies in camelids. In Proceeding of the Annual Convenetion of the American Association of Bovine Practitioners,Charlotte NC ?, 177-184, 2008.
Evaluation of Passive Transfer of Immunity
The epitheliochorial microcotyledonary placenta of camelids does not allow passage of immunoglobulins from the dam to the fetus. Therefore, newborn camelids are born hypogammaglobulinemic and rely on passive transfer of immunity through colostrum intake.9 Increased incidence of illness and death from infections in neonates is associated with inadequate passive transfer of immunoglobulin as measured by low serum immunoglobulin concentrations in sick or dead neonates.10 Conversely, successful immunoglobulin transfer is associated with low infection rates and the high likelihood of survival. Even though this is true, great differences exist among farms with regard to immunoglobulin concentrations in healthy neonates. The effect of between-farm variations, which could involve factors such as management practices, including biosecurity measures, nutrition, vaccination programs, current pathogen history, herd bloodlines, geographic location, and climate, would enable a neonate with failure of passive transfer to remain healthy.
A relative paucity of research is seen in the area of colostrogenesis in camelids. Concentration of immunoglobulin G (IgG) in mammary secretion is 8 to 10 times higher than that in serum about 3 weeks before parturition.11 However, data indicate that camelids do not selectively transfer immunoglobulin (specifically IgG1) from serum to the mammary gland prior to parturition.11 Although Bravo et al. have suggested that periparturient camelids may produce IgG in the mammary gland, this has not been fully investigated. The concentration of IgG drops quickly in mammary secretion after parturition.11
A strategy for colostrum supplementation should be immediately instituted upon discovery of any problem with regard to colostrum production or intake. The best approach is to provide colostrum from the same species. If this is not possible, the second best choice is goat colostrum.12 It is important that the source of noncamelid colostrum be free from major infectious diseases (i.e., bovine viral diarrhea virus [BVDV], Johne disease, brucellosis, etc.). Cattle colostrum is the second best choice for supplementation in crias. Powdered colostrum supplements are available for some species but have not been critically evaluated in camelids.
As a general rule, colostrum administration should be started immediately in cases of agalactia or mastitis or if the newborn has not been seen suckling by 2 hours after birth. Crias should receive 10% of their body weight in the first 12 hours of life divided into meals every 2 hours. This usually represents about 60 to 90 mL every 2 hours. Some practitioners prefer to give 5% of body weight in a feeding followed by another feeding of equal quantity 6 to 8 hours later because small intake volumes may accelerate gut closure for colostrum. The best way to administer colostrum is by bottle-feeding. In case of poor suckling reflex, colostrum may be administered via orogastric intubation (24-French). Care should be taken not to place the tube in the respiratory tract (trachea). The tube should be felt by palpation as it goes down the left side of the neck. It should be kept within the esophagus to provide closure of the esophageal groove and avoid depositing milk within the first stomach compartment, which would increase the risks of fermentation. Repeated tube feeding may cause esophagitis.13
Poor IgG status of the newborn is the most important risk factor for neonatal infectious diseases. Most insurance companies require determination of the passive immunity status for crias. The concentration of IgG increases rapidly after colostrum ingestion and reach a peak between 24 and 48 hours of age. Therefore, determination of IgG levels in serum is ideally performed 24 to 36 hours after birth. Methods used for evaluation of passive transfer of IgG include serum radial immunodiffusion (SRID), serum total solids measurement using a refractometer, serum total protein, globulin concentration, and sodium sulfite turbidity test.
SRID is the gold standard for evaluation of passive immunity status in the newborn alpaca and llama. This technique is quantitative, specific, and highly accurate.14–16 Since the SRID is species specific, it will not be accurate, if colostrum other than that of camelids was used. Using Triple J Plates (Triple J Farms, Bellingham WA) levels greater than 800 mg/dL are considered adequate, and levels less than 400 mg/dL are considered an indication of failure of passive transfer. However, most studies consider 1000 mg/mL to be the minimum cutoff value for adequate passive transfer.14,15
Failure of passive transfer should be suspected if total solids determined by refractometer are less than 4.5 g/dL, whereas values greater than 5.5 g/dL are consistent with adequate passive transfer.14 However, others have found that this test is not very accurate.17 If samples are taken at birth and compared with samples at 24 hours, the packed cell volume (PCV) will be observed to be reduced and the total solids increased, indicating that the cria is more hydrated and that the rise of total protein is caused by significant passive transfer. Serum total protein and globulin should be at least 5.5 g/dL and 2 g/dL, respectively.17 However, it is important to remember that these parameters may be falsely increased in case of dehydration. Commercial sulfate turbidity tests are available but not as sensitive as the SRID.14
Prophylactic Treatments of the Newborn
The most common infections of newborn camelids in the first few days of life are clostridial diseases caused by Escherichia coli and coronavirus. Clostridiosis caused by Clostridium perfringens type A has been reported to cause severe losses among some populations of crias.18,19 Protection of the cria against these infections is provided by adequate ingestion of colostrum and absorption of IgG from a vaccinated dam. Although several practitioners prefer not to vaccinate pregnant females, we have not seen any problems with administration of C. perfringens type C and D and C. tetani vaccine in the last 6 weeks of pregnancy. Administration of clostridial antitoxin should be considered if the cria did not have adequate passive transfer. Vaccination of crias in their first week of life has been proposed by some authors. Autologous vaccines may help reduce the effects of C. perfringens type A.
Recently, alpaca breeders have been giving bovine modified live vaccines against coronavirus orally in the first 24 hours of life. This strategy has been reported to be effective in controlling coronavirus outbreaks, probably because of the close genetic relationship between the strains isolated from alpacas and those from cattle.20 The use of these vaccines in pregnant camelids to enhance colostral protection has not been fully investigated in alpacas and llamas. Abortion has been reported following parenteral use of these vaccines.
Crias should receive vitamins A, D, and E, as well as selenium, in areas where needed. Crias that do not receive vitamin D supplementation have reduced growth rate during winter and may show clinical signs of rickets if born in winter.21 If local conditions justify, a dose of 1000 international units (IU) of D3 per kilogram of body weight subcutaneously has been suggested for crias in late autumn and again in midwinter and to adult females in midwinter to prevent vitamin D inadequacy.22
It has become a standard practice in North America to test all newborn SACs for BVDV (polymerase chain reaction [PCR] test) for early identification of persistently infected (PI) crias so that they can be separated from the herd.23–25
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


