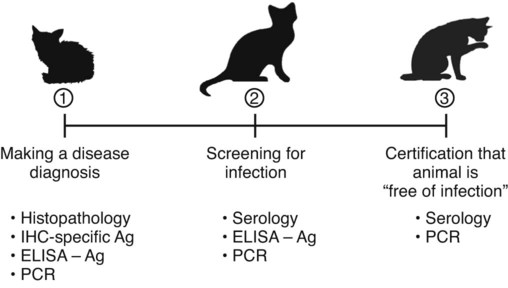
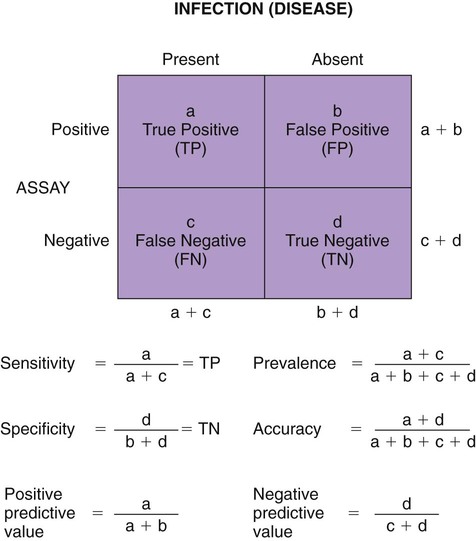
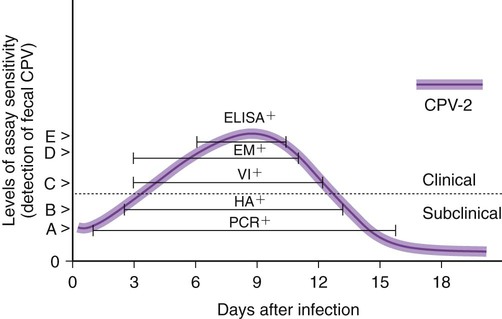
The detection of viral and rickettsial infections is of vital importance as our range of clinical inquiry continues to expand. In addition to recognizing clinical manifestations associated with particular viral and rickettsial organisms, clinicians are being requested to certify animals “free of infection,” screen animals for particular infections, and be aware of emerging infections that can occur via random mutation or interspecies transmission. Clinical inquiry generates information that not only clarifies the disease status of an animal, but also determines whether an infectious microbe is present and in what manner it is being shed (Table 1-1). Once the presence of infection is confirmed, then treatment regimens can be formulated, and control steps for containing the infection/disease process can be taken. This includes, if available, appropriate vaccination of susceptible animals. TABLE 1-1 Interpretation of Laboratory Analysis of a Suspect Case of Canine Parvoviral Enteritis CCV, Canine coronavirus; CPV, canine parvovirus; ELISA, enzyme-linked immunosorbent assay; EM, electron microscopy; HI, hemagglutination-inhibition; PCR, polymerase chain reaction. A case in which canine parvoviral enteritis was suspected or being monitored based on clinical signs or at-risk category. Interpretation depends on the level of clinical inquiry and types of laboratory tests used. There are various diagnostic assay technologies available for detecting viral and rickettsial infections of dogs and cats.15,33,41,42,87 In some cases, diagnosing disease is the veterinarian’s primary goal so that an appropriate course of action can be implemented (e.g., diagnosing canine herpesvirus in a litter of pups with fading puppy syndrome). In other situations, it may be important to determine the infection status of a cat with feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) before entry into a cattery. As assays have developed, testing strategies have evolved to assist the veterinarian. The use of serologic assays for determining the immune status of dogs and cats has become increasingly popular as the need for annual vaccine boosters is evaluated.11 The use of molecular diagnostics has allowed polymerase chain reaction (PCR) to be applied at a screening test where several infectious agents are probed for simultaneously.49 Other areas important to companion animal veterinarians include the application of clinical epidemiology as it relates to infection and disease prevalence, multiple-animal households, population-based biosecurity measures in humane facilities, the potential for interspecies spread of infections between dogs and cats, and pet-associated disease in humans (zoonosis).8,59 As diagnostic assays become more sensitive, the need to establish clinical relevance becomes paramount to our pursuit of the clinical utility of an assay. Earlier reports emphasized the importance of differentiating the detection of an infection from the diagnosis of disease.33 This distinction continues to be of primary importance to clinicians when making decisions regarding the most appropriate time to collect samples, the most appropriate sample to collect, and the best assay to perform to maximize the chance of a meaningful result. This series of questions constitutes the most important aspect of making a diagnosis. Veterinarians use diagnostic testing for three main reasons: (1) to diagnose an acute or chronic infectious disease process, (2) to detect a subclinical infection when susceptible animals or humans may be vulnerable to infection and/or disease, and (3) to certify animals as being not only “disease free,” but also “free of infection” (Fig. 1-1). As expectations of preventative veterinary medicine have increased so has the demand for early and accurate detection of infections.24 Although the majority of diagnostic assays have been validated to assist diagnosing a specific infection (e.g., the canine parvovirus [CPV] antigen enzyme-linked immunosorbent assay [ELISA] on feces), more clinicians are utilizing PCR which enables the diagnostician to detect multiple infectious agents simultaneously (See Molecular Diagnostics).49,59,59 The use of PCR has added a new dimension of sensitivity for detecting the latent phases (host infected, but no virus actively replicating) of herpesvirus and retrovirus infections, such as feline herpesvirus (FHV), FeLV, and FIV infections.53,59,59 It has also allowed clinicians to recognize the importance of carrier animals in the population, such as feline enteric coronavirus (FECV) and the newly recognized canine respiratory coronavirus infections.1,9,9 This allows the veterinarian to make some expanded decisions at the individual level (e.g., prognosis, to medicate or not, or to vaccinate a particular animal), and at the population level (vaccinate, segregate, or depopulate). There is an increasing trend to use diagnostic tests in nonclinical situations as previously mentioned. These can include their use in surveillance, estimating infection prevalence in a practice area, and performing risk factor analysis.4,5,12,43 The testing of apparently healthy animals is becoming a standard biosecurity tool in such cases as before a show, sale, adoption, breeding, or placement in high-risk human environments (such as children’s classrooms, hospitals, and convalescent care centers), which may require an animal to be certified not only “disease free,” but also “free of infection” with respect to a carrier state.24 Although the goal of a diagnostic assay is 100% accuracy, it is important to recognize that laboratory tests have varying degrees of sensitivity, specificity, and fit for purpose.57 This has established a diagnostic hierarchy, which is used by clinics and diagnostic laboratories. An example of this type of hierarchy is presented in Fig. 1-1 when displaying the different settings in which an assay may be called on. An example would be when screening a cattery for FIV infection to certify it “free of infection.” Initially a serologic assay would be used to screen the population. If all cats have seronegative test results, then PCR would be used to confirm the negative status and certification would be granted. Test sensitivity is the likelihood that an animal known to have a particular disease or infection with a particular microbe will be identified with a positive test result (true positive) (Fig. 1-2). A test with high sensitivity has few false-negative results. The limits to test sensitivity can extend to an animal with a subclinical infection (i.e., latency, asymptomatic carrier, or early during the incubatory phase of disease); therefore, the values vary for either disease or infection. When making a diagnosis of CPV infection it is important to recognize that although some PCR assays are more sensitive than antigen-detection ELISA (Fig. 1-3), their ability to accurately diagnose CPV disease may be compromised because they can detect subclinically infected dogs as well as diseased dogs. Conversely, the CPV-antigen ELISA is very good for detecting CPV-associated enteritis, but not sensitive enough to detect CPV in dogs that are subclinically infected.33 Test specificity is the likelihood that an animal known to be free of disease or infection will have a negative test result.43,86 A highly specific test has few false-positive results. Knowing the specificity of a diagnostic test is very important because more false-positive results occur with a test of low specificity. Fit for purpose means that test methods must be appropriate for specific diagnostic applications in order for the test results to be of any clinical relevance.57 This has become an essential prerequisite for diagnostic assays, whether they are conducted in the clinic setting or at a regional diagnostic laboratory. As testing strategies evolve, firm recommendations for the expectations and limitations of diagnostic assays will become evident.24 Because an assay may not be able to distinguish an animal that is subclinically infected from an animal with disease, a test’s predictive value is very important to the veterinarian (see Fig. 1-2). Classically, a positive predictive value is the probability that an animal with a positive test result truly has the disease. A negative predictive value is the probability that an animal with a negative test result does not have the disease. These predictive values can also be used for determining the clinical utility of detecting latency in a population or a subclinical carrier as long as the assay is fit for that purpose. The predictive values depend on the prevalence of the disease in the regional population.33,43 Laboratory diagnosis of viral and rickettsial infections requires proper sample selection, collection, and preservation. As noted earlier, there are three main situations in which obtaining a diagnosis is of value (see Fig. 1-1), and sample selection would vary according to what level of clinical inquiry the veterinarian is targeting. In order to make a definitive disease diagnosis, it is best to collect samples during the acute phase of the disease. During this phase, the microbe is usually at its highest concentrations in areas such as body fluids, excretions, secretions, or blood.7,37,37 Samples can be collected antemortem, or, if necessary, at necropsy (Table 1-2). Antemortem samples can be collected noninvasively, such as oropharyngeal swabs for feline calicivirus (FCV), or invasively, such as the case of cerebrospinal fluid for canine distemper virus (CDV) diagnosis. Samples can be valuable in the management of other animals in the susceptible population; however, the degree of diagnostic accuracy is generally reduced because of microbial protein and nucleic acid degradation. For this reason fresh samples should be promptly refrigerated for short-term shipment (12 to 48 hours) to a laboratory close to the clinic or by overnight delivery service. For long-term shipments (2 to 4 days), samples should be frozen and shipped on wet ice to the laboratory. Tissues obtained after biopsy or necropsy for histopathology and immunohistochemistry (IHC) should be promptly fixed in buffered formalin. Serology can be of diagnostic value if a single sample is available for IgM analysis, or acute and convalescent sera are available for IgG analysis. After samples have been collected, specimen preservation and shipment to the testing laboratory become important for rapid analysis and turnaround times. Table 1-3 gives guidelines for specimen collection, processing, and shipment for laboratory diagnosis. Because of the changing nature of diagnostic assays and the hierarchy of sensitivity and specificity, it is recommended that the laboratory be consulted for the optimum sample collection, processing, and shipment protocols. Web Appendix 5 lists those laboratories that conduct tests for infectious diseases of dogs and cats. TABLE 1-2 Sample Collection for Laboratory Diagnosis of Viral and Rickettsial Diseases aSamples should be kept moist and chilled. cSample should be collected in EDTA and kept refrigerated. dHematogenous organs: lung, liver, kidney, bone marrow, and spleen. eAn animal with neurologic signs should be handled with extreme caution; before further diagnostic testing is performed, a public health laboratory should state that the animal is free of rabies virus (see Chapter 20). Modified from Ref. 72. TABLE 1-3 Specimen Collection, Processing, and Shipment for Diagnosis of Viral and Rickettsial Diseases CDV, Canine distemper virus; ELISA, enzyme-linked immunosorbent assay; FA, fluorescent antibody; FBS, fetal bovine serum; PCR, polymerase chain reaction. aCollected in EDTA and kept refrigerated. bUse Universal Viral Transport Media (#220221, Becton Dickinson Co, Sparks, MD). The diagnosis of viral infections revolves around five different techniques, which have varying degrees of sensitivity and specificity.7,33,60,65 These include (1) virus isolation in cell culture, (2) electron microscopy, (3) specific viral antigen detection by immunologic methods (ELISA, fluorescent antibody, immunoperoxidase, and IHC), (4) nucleic acid detection (PCR and in situ hybridization), and (5) serologic testing for specific viral antibodies. These assays have a hierarchy of use as previously mentioned and depicted in Fig. 1-1. Although nucleic acid–based PCR assays are increasingly available through commercial diagnostic laboratories for the detection of common infectious diseases of dogs and cats (see Table 1-3 and Web Appendix 5), other diagnostic techniques still play important roles in diagnosis because of availability, reliability, and cost. In certain situations or time points during the course of infection, PCR may be insensitive. The results of PCR are often best interpreted in conjunction with the results of serologic assays. The in-clinic ELISA assays for detection of FeLV antigenemia in cats with regressive and progressive infection and for diagnosis of CPV-2 strains in dogs with enteritis have been major contributors to control of these diseases in companion animals. Specialized diagnostic laboratories should be consulted for more extensive analysis of specimens from a particular case if the number of clinically ill animals significantly increases, unusual clinical signs are observed, or new variants of preexisting viruses such as CPV, CDV, and FCV are suspected. Veterinarians should remain vigilant for emerging infectious diseases, which may express themselves in immunocompromised dogs and cats (e.g., influenza in shelter populations; see Chapter 23), and in the general population as well.23,59 Virus isolation and PCR have been primarily used to detect previously unidentified or new strains of viruses and rickettsia.* See respective chapters (Chapters 3 to 24) for additional information on specific viral infections of dogs and cats. Rickettsiae are small, obligate, intracellular gram-negative bacteria that are included with viruses because of the common methods that are used to diagnose infections caused by them.33,41,41 Rickettsia generally require living cells for propagation and are usually cultured in embryonating chicken eggs or in cell culture. See Chapters 25 to 27. This is similar to isolation of Chlamydia; see Chapter 28. Because of their fastidious nature, serology has been very valuable in the diagnoses of rickettsial infections. Just as the clinical use of PCR has improved the ability to diagnose viral infections, there has been increased use of this technique for detection of rickettsial infections as well.73,87 Rickettsiae of veterinary importance and commonly used diagnostic assays are listed in Table 1-4. TABLE 1-4 Rickettsial Infections of Veterinary Importance
Laboratory Diagnosis of Viral and Rickettsial Infections and Clinical Epidemiology of Infectious Disease
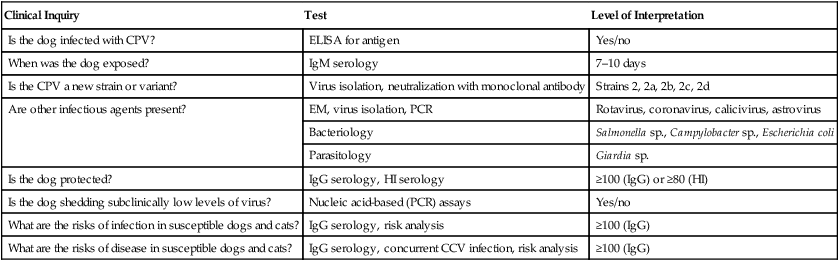
Clinical Inquiry
Test
Level of Interpretation
Is the dog infected with CPV?
ELISA for antigen
Yes/no
When was the dog exposed?
IgM serology
7–10 days
Is the CPV a new strain or variant?
Virus isolation, neutralization with monoclonal antibody
Strains 2, 2a, 2b, 2c, 2d
Are other infectious agents present?
EM, virus isolation, PCR
Rotavirus, coronavirus, calicivirus, astrovirus
Bacteriology
Salmonella sp., Campylobacter sp., Escherichia coli
Parasitology
Giardia sp.
Is the dog protected?
IgG serology, HI serology
≥100 (IgG) or ≥80 (HI)
Is the dog shedding subclinically low levels of virus?
Nucleic acid-based (PCR) assays
Yes/no
What are the risks of infection in susceptible dogs and cats?
IgG serology, risk analysis
≥100 (IgG)
What are the risks of disease in susceptible dogs and cats?
IgG serology, concurrent CCV infection, risk analysis
≥100 (IgG)
Clinical Relevance
Diagnostic Assay Interpretation
Sample Selection and Preservation
Sites or Clinical Signs
Antemortem Samplesa
Postmortem Samplesb
Respiratory, oral, and ocular tissues
Nasal, ocular, pharyngeal swabs; conjunctival scraping; serum; whole bloodc; transtracheal wash
Selected tissuesd and bronchiolar lymph nodes
Gastrointestinal tract
Feces, vomitus, serum, whole bloodc
Selected small intestine sections, intestinal contents, mesenteric lymph nodes
Skin and mucous membranes
Vesicle fluid, swabs, scrapings of lesions, serum, whole bloodc
Selected tissuesd and regional lymph nodes
Central nervous systema,e
Cerebrospinal fluid, serum, whole bloodc, feces
Selected brain sections
Genitourinary tract
Urogenital swabs, vaginal mucus, urine, serum, whole bloodc
Selected sections from placenta, fetal lung, liver, kidney, and spleen
Immunosuppression, hematologic abnormalities, blood dyscrasias
Whole bloodc, serum, bone marrow
Selected tissuesd and lymph nodes
Procedure and Specimen
Collection and Processing
Shipment
Organism isolation, nucleic acid-based testing (PCR), ELISA for antigen
Specimens: Tissue, excretions, secretions
Collect aseptically to prevent bacterial contamination, and store at ≤10° C to prevent inactivation; do not freeze or fix.
Use whole blooda, tissue biopsy, feces, swabs, commercial transport media.b
Serology
Specimens: Serum, cerebrospinal fluid, synovial fluid
Collect aseptically to prevent contamination, and handle gently to prevent hemolysis; remove needle from syringe before dispensing; allow to clot at room temperature; rim clot and centrifuge at 650 g for 20 min; pipette serum fraction into clean tube; although paired samples (10–14 days apart) are preferred, single samples can be diagnostic (e.g., CDV IgM).
Refrigerate until shipping; double bag for shipment; use leak-proof vial.
Histology, immunohistochemistry
Specimens: Tissue
Collect aseptically to prevent contamination, 5 mm thick; fix in 10% buffered formalin (10 × volume).
Double bag for shipment; ship in leak-proof container with adequate fixative.
Direct FA testing
Specimens: Tissue, tissue impression
Make tissue impression on clean, dry microscope slide and air dry; fix in alcohol for cytology or in acetone for direct FA.c
Pack on wet ice and ship as for isolation; smears can be shipped unrefrigerated.
Electron microscopy
Specimens: Tissue
Collect aseptically, 1 × 2 mm thick; fix in 2%–4 glutaraldehyde (10 × volume) for 24 hr at 20° C.
Double bag for shipment; ship in leak-proof container with adequate fixative.
Feces or body fluids
Collect fresh; do not freeze or fix.
Refrigerate until shipping; double bag for shipment; pack on wet ice to last 48–72 hr.
Laboratory Analysis
Viral Infections
Rickettsial Infections
Agent
Host
Disease
Diagnosis
Neorickettsia helminthoeca and Elokomin fluke fever agentsa
Dogs, coyotes, foxes, ferrets
Salmon poisoning and salmon fever
Observation of fluke eggs (Nanophyetus salmincola) in feces
Demonstration of the agent in lymph node aspirates
Ehrlichia spp.b
Humans; dogs, cats, and other domestic animals
Ehrlichiosis
Indirect FA test for antibody in serum
Giemsa-stained blood smears or marrow
PCR on whole blood
Rickettsia spp.c
Humans, dogs, cats
Rocky Mountain spotted fever
Indirect FA test for antibody in serum
Giemsa-stained blood smears
PCR on whole blood
Mycoplasma haemofelisd
Cats
Feline infectious anemia
Giemsa- or FA-stained blood or tissue smears; presence of agent on red blood cells inconsistent ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Laboratory Diagnosis of Viral and Rickettsial Infections and Clinical Epidemiology of Infectious Disease