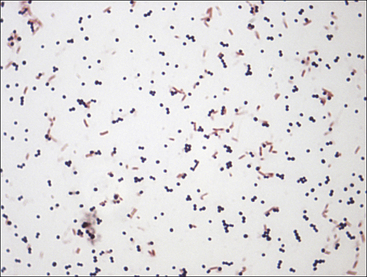
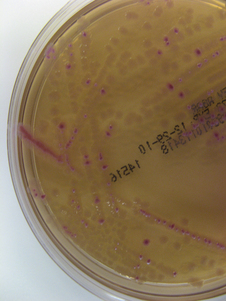
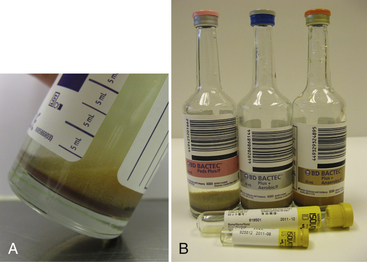
Chapter 3 • Because of the increasing prevalence of antimicrobial drug resistance among bacteria isolated from dogs and cats, culture and susceptibility is always recommended when a bacterial infection is suspected at a normally sterile anatomic site. • Successful isolation of aerobic and anaerobic bacteria from a clinical specimen relies on collection of a sufficient quantity of material, from the correct location, and appropriate storage and transport of the specimen to the laboratory. Care should be taken to prevent specimen contamination during collection. • The laboratory should be provided with an appropriate history if fastidious, anaerobic, or slow-growing bacterial organisms are suspected. • Concurrent examination of a Gram-stained smear by the laboratory assists in assessment of specimen quality and interpretation of culture results. • Antimicrobial susceptibility testing is most commonly performed using broth microdilution or agar diffusion testing and aims to determine the minimum concentration of an antimicrobial drug that inhibits growth of the organism in vitro (minimum inhibitory concentration, or MIC). The laboratory uses MIC breakpoints, which are established and revised regularly by national standards agencies, to assess the susceptibility of an organism to a particular antimicrobial drug. 1. An appropriate level of suspicion for the presence of a bacterial infection; 2. Development of a list of differential diagnoses that reflects the types of bacterial pathogens that might be the cause of clinical signs, because of the requirement of some bacteria for special transport conditions, culture conditions, or prolonged incubation; 3. Collection of a sufficient amount of specimen; 4. Collection of the correct type of specimen; 5. Specimen collection from the correct anatomic location; and 6. Proper handling, storage and transport of the specimen, which should be sent with a request for culture to the laboratory within acceptable time limits. Veterinary clinicians should collect specimens for culture using an appropriate method from the correct anatomic site. Collection of a sufficient amount of specimen is very important. Table 3-1 suggests optimal specimen types for detection of bacteria from specific anatomic sites. Attempts should always be made to prevent contamination with commensal bacteria during specimen collection. When anaerobes are suspected, aspirates or biopsies should be obtained rather than swabs, and the specimen should be stored at room temperature and not refrigerated. This is because oxygen diffuses into cold specimens more rapidly than specimens held at room temperature. TABLE 3-1 Suggested Specimen Types for Bacterial Culture from Selected Anatomic Sites and Likely Pathogens Present Specimens should be transported to the laboratory immediately. As a general rule for aerobic culture, if transport requires longer than 2 hours, appropriate transport medium should be used or specimens should be refrigerated without transport medium. Although specimens can be held for up to 72 hours, culture of specimens older than 24 hours should be avoided, even if they have been refrigerated or placed in transport media, because of increased likelihood of false positive and false negative results with longer storage times. Transport medium for bacterial culture is usually supplied in a plastic tube with one or two swabs attached to the tube’s cap (Figure 3-1). The medium is designed to maintain bacterial viability without causing significant growth. It usually consists of a small amount of agar, reagents that maintain pH, a colorimetric pH indicator that indicates whether oxidation has occurred, and specific factors that maintain the viability of certain pathogens. Some organisms, such as chlamydiae and leptospires, have specific transport medium requirements. Specimens that are placed in transport media should not be refrigerated. Specimens should be labeled with the patient’s name, patient number, source of specimen, and the date and time of collection, and should be labeled, packaged, and transported according to regional and national regulations (see Chapter 1). Specimens for anaerobic culture should preferably be transported in an oxygen-free container. Microscopic Examination of Direct Smears A Gram stain prepared from the specimen can permit the rapid preliminary diagnosis of infection and determine whether the organism(s) present are gram-positive or gram-negative (Box 3-1; Figure 3-2). This helps guide the clinician to select an appropriate empiric therapy, if necessary, while awaiting the results of culture and susceptibility testing. If sufficient material is available, examination of a direct smear also helps to determine whether a specimen is adequate for culture and aids interpretation of culture results. For swab or aspirate specimens, clinicians should consider providing a separate specimen for a direct smear in addition to a specimen for culture. FIGURE 3-2 Gram-stained smear of gram-positive cocci (dark blue) and gram-negative rods (red) (1600× oil magnification). Other microscopic techniques used for bacterial identification in specialized laboratories include darkfield microscopy, phase-contrast microscopy, and fluorescence microscopy (see Chapter 2). Darkfield and phase contrast microscopy allow visualization of organisms without fixation and staining, so motility as well as structure can be assessed. In darkfield microscopy, the condenser only permits light that bounces off an object to pass from below up into the objective, so the object appears bright against a dark background. Darkfield microscopy is used primarily for identification of fine spirochetes such as leptospires, which cannot be visualized using conventional microscopy (see Chapter 50). In phase-contrast microscopy, slowing and deflection of beams of light occur as they pass through an object. The performance of bacterial culture for diagnostic purposes requires appropriate laboratory facilities, proper biosafety level-2 containment, properly trained individuals, reference strains for quality control testing, and the use of standard protocols.5 Media used for bacterial culture may be categorized as general-purpose, enriched, selective, differential, and specialized media (Table 3-2). Some media belong to more than one of these categories. For example, MacConkey agar is both a selective and a differential medium (Figure 3-3). Media for culture contains a nutrient source, most commonly peptones (hydrolyzed animal or vegetable substances), and is adjusted to a specific pH. Solid medium contains agar, which is derived from seaweed. Other nutrients may be added based on specific organism requirements. More than 100 different types of bacteriology media exist, most of which can be purchased as preprepared plates or broth. TABLE 3-2 Types of Media Used in Bacteriology FIGURE 3-3 Growth of bacteria on MacConkey medium that shows the selective and differential qualities of the medium. The medium contains substances that inhibit the growth of gram-positive bacteria. By using the lactose available in the medium, bacteria such as Escherichia coli, Enterobacter spp., and Klebsiella spp. produce acid when they grow on the medium, which lowers the pH of the agar and results in the formation of red to pink colonies. Blood culture media contain the anticoagulant sodium polyanethol sulfonate (SPS), which is less inhibitory to bacterial growth than other anticoagulants. Some blood culture media (e.g., BD BACTEC Plus) contain resin beads that bind antimicrobial drugs in the patient’s blood specimen, so they do not inhibit bacterial growth (Figure 3-4). The detection of bacterial growth in blood culture systems can be automated using instrumentation that measures emission of fluorescence or a colorimetric change in a sensor. The change occurs as CO2 concentrations within the bottle increase, which indicates the presence of bacterial growth. Some instruments detect pressure changes in the head of the bottle. The culture is monitored at regular intervals by the instrument, to optimize early detection of bacterial growth. Positive blood culture bottles are then evaluated by performing a Gram stain of the broth, and subculture to appropriate media. FIGURE 3-4 Blood culture systems. A, BACTEC system showing resin beads that bind antimicrobial drugs. B, Pediatric, aerobic, and anaerobic BACTEC systems for blood culture together with adult and pediatric WAMPOLE Isolator tubes (front) for lysis-centrifugation culture of blood. Another blood culture system is the Isolator lysis centrifugation system (DuPont, Wilmington, DE) (See Figure 3-4, B). In this system, blood is added to a tube that contains anticoagulant and a reagent that induces RBC lysis. The tube is centrifuged, and the pellet is plated onto agar media and incubated. This method allows colony counts to be obtained. The tubes allow isolation not only of aerobes, but also some anaerobic bacteria. In the laboratory, media for bacterial culture should be warmed to room temperature before inoculation, and damaged media, such as dehydrated medium or medium that has changed color, should not be used. Medium is stored in the dark at 2°C to 8°C and discarded when it reaches its expiration date. After plating, incubation is most commonly performed at 35°C to 37°C in air for a minimum of 2 days. Conditions vary for specific bacteria, and growth in 3% to 5% CO2 is preferred by some fastidious organisms. The laboratory should be informed, by providing an adequate clinical history, when pathogens with special culture requirements are suspected, such as Campylobacter, Mycoplasma, Actinomyces, Nocardia, and Mycobacterium spp. These organisms require special media or prolonged incubation times (e.g., weeks). Specimens for anaerobic culture should be plated onto appropriate media immediately and incubated in an anaerobic environment. Anaerobic containers, which create an anaerobic environment through the use of gas-generating reagents, can be used for incubation (Figure 3-5). Because anaerobes grow more slowly than aerobes, anaerobic cultures should be held for 7 days before being reported as negative. FIGURE 3-5 System for isolation of anaerobic and microaerophilic bacteria. The packets are opened, and the sachets inside are placed into the container with the inoculated plates. The container is sealed immediately. The sachets contain ascorbic acid. Atmospheric oxygen in the container is rapidly absorbed and replaced with carbon dioxide in an exothermic reaction process. After an appropriate incubation period, the container is opened and the plates inspected for growth. An anaerobic indicator can be placed within the box to ensure that the proper conditions are maintained.
Isolation and Identification of Aerobic and Anaerobic Bacteria
Introduction
Specimen Collection and Transport
Specimen Type
Suggested Volume for Culture
Likely Pathogens Present
Blood
≥10 mL for patients weighing >10 kg, or 1% of patient blood volume
Gram-positive and gram-negative aerobes. Common contaminants are coagulase-negative staphylococci, Corynebacterium, Bacillus spp., and propionibacteria (see Chapter 86)
CSF
≥0.5 mL
Gram-positive and gram-negative aerobes, anaerobes, Brucella spp. (dogs), Nocardia, Actinomyces,1 Mycoplasma spp. (cats)2 (see Chapter 90)
Middle ear
Aspirate of fluid or pus through tympanic membrane
Gram-positive and gram-negative aerobes, anaerobes, Mycoplasma spp., Actinomyces spp.2,3 (see Chapter 84)
Skin
Swab from epidermal collarette or ruptured pustule (superficial pyoderma); biopsy from which dermis has been removed (deep pyoderma)
Gram-positive aerobes (especially staphylococci), rarely Pseudomonas aeruginosa (see Chapter 84)
External ear
Swab ear canal after removing debris and crusts from the canal
Gram-positive aerobes, Pseudomonas aeruginosa (see Chapter 84)
Cornea
Scrape or swab exposed corneal stroma and inoculate directly onto media. Referral to veterinary ophthalmologist recommended
Gram-positive aerobes, Mycoplasma spp., Pseudomonas aeruginosa4
Abdominal fluid
1-5 mL
Gram-negative and gram-positive aerobes, anaerobes, Actinomyces spp. (see Chapter 88)
Pleural fluid
1-5 mL
Gram-negative and gram-positive aerobes, anaerobes, Actinomyces spp., Nocardia spp., Mycoplasma spp. (see Chapter 87)
Feces
Fresh whole stools preferred. Avoid swabs or fecal loops containing fecal matter due to low specimen size. Submit in clean, dry leak-proof container.
Escherichia coli, Salmonella, Clostridium perfringens, Clostridium difficile, Campylobacter spp., Anaerobiospirillum (Chapters 45–49 and 88)
Urine
1-5 mL urine collected using cystocentesis
Gram-negative and gram-positive aerobes, Corynebacterium urealyticum (see Chapter 89)
Bronchoalveolar lavage specimens
1-5 mL
Gram-positive and gram-negative aerobes, anaerobes (aspiration pneumonia), Mycoplasma spp., rarely Mycobacterium spp. (see Chapter 87)
Diagnostic Methods
Bacterial Culture
Medium Type
Attributes
Example
Transport
Nonnutritive liquid or semisolid medium. Promotes organism viability without allowing significant growth.
Amies transport medium
General-purpose
Allows growth of most aerobic and facultatively anaerobic organisms
Sheep blood agar
Enriched
Promotes growth of fastidious organisms that require specific nutrients, for example Francisella
Chocolate agar, which is enriched with heat-treated blood, which gives it a brown color
Selective
Selects for growth of certain organisms while inhibiting others
MacConkey agar, which selects for gram-negative bacteria
Differential
Aids in preliminary identification of organisms based on colony appearance
MacConkey agar, which promotes pink colony formation by organisms that ferment lactose
Specialized
Selects for a specific pathogen through the addition of specific nutrients
Anaerobic medium, which contains hemin, vitamin K, and reducing agents

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Isolation and Identification of Aerobic and Anaerobic Bacteria
Only gold members can continue reading. Log In or Register to continue