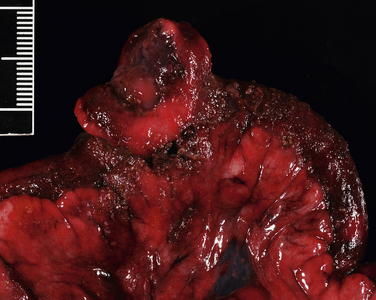
Chapter 88 Gastroenteritis in dogs and cats may be caused by an enormous array of microorganisms that include viral, bacterial, fungal, and protozoal pathogens (Table 88-11–5). Other parasites (such as nematodes) should also be considered on the differential diagnosis list. Enteropathogenic bacteria cause diarrhea by adhesion to or destruction of enterocytes, secretion of a variety of potent enterotoxins, and stimulation of the host inflammatory response. Many of these microbes can be detected in the feces of apparently healthy animals as well as the feces of those with clinical signs of diarrhea. Therefore, it may be difficult to ascertain the role that these organisms play in disease in a single patient. Clinical signs of diarrhea may be more likely to occur when multiple organisms are present simultaneously (“polyparasitism”). In addition, other host factors (such as nutritional status or age) and bacterial virulence factors influence whether clinical signs develop. When outbreaks occur in dog and cat populations, collection of specimens from multiple affected and in-contact animals may be useful to determine the significance of one or more organisms involved. Use of antibiotics to treat bacterial diarrhea should be reserved for animals with systemic signs of illness such as fever, lethargy, and leukocytosis on the CBC.6 Other infections are self-limiting, and the mainstay of treatment is proper fluid therapy and supportive care. TABLE 88-1 Examples of Potential Infectious Causes of Enterocolitis in Dogs and Cats The reader is referred to other chapters in this book for detailed information on pathogenesis, clinical signs, diagnosis, and treatment for the organisms that are most commonly involved. References are also provided in Table 88-1 for information on pathogens that are uncommon or rarely identified or that have uncertain pathogenicity. In humans, primary peritonitis has also been referred to as spontaneous bacterial peritonitis and most often complicates development of ascites (e.g., secondary to cirrhosis, hepatitis, or congestive heart failure).7,8 In contrast, conditions that predispose to ascites are rarely present in the history of dogs and cats with primary peritonitis. Some primary peritonitis in dogs or cats may result from hematogenous spread of bacteria to the peritoneum or bacterial translocation from the gastrointestinal tract.7 Secondary peritonitis is the most common form of peritonitis in dogs and cats and occurs when bacteria are introduced into the peritoneal space as a result of gastrointestinal perforation, penetration of the abdominal wall, rupture of the genitourinary tract, rupture of intra-abdominal abscesses, gallbladder rupture, or ascending infection of the umbilicus in neonates (Table 88-2).7,9–15 In dogs, low preoperative total protein or serum albumin concentrations are risk factors for development of bacterial peritonitis after gastrointestinal surgery.16 TABLE 88-2 Underlying Causes of Secondary Peritonitis in Dogs The bacterial species involved in peritonitis reflect the normal flora of the gastrointestinal tract. Mixed aerobic-anaerobic infections, with up to four different bacterial species, occur in more than 50% of affected dogs and cats.7,10 Escherichia coli is the most common isolate from both dogs and cats, followed by Enterococcus and Clostridium spp.7,10,11,14,15,17,18 Other isolates include staphylococci, streptococci, Pseudomonas aeruginosa or Acinetobacter spp., a variety of anaerobes, gram-negative Enterobacteriaceae (Proteus, Citrobacter, Serratia, Klebsiella, or Enterobacter), Actinomyces, or in cats, Pasteurella multocida. In one study, a greater proportion of dogs with primary peritonitis had gram-positive bacterial infections than dogs with secondary peritonitis.7 Uncommonly, Candida albicans can be involved, especially if there is a history of antibiotic treatment (see Chapter 67). The mean age of dogs with peritonitis is around 5 to 7 years, and the mean age of cats is 7 to 10 years, although dogs and cats of any age can be affected.9,12,19 Age was not found to relate to development of primary versus secondary peritonitis in dogs or cats.7 There is no known breed or sex predisposition. In one study, three quarters of affected cats were indoor-only.10 The most common historical signs in dogs and cats with bacterial peritonitis are lethargy, anorexia, vomiting, and diarrhea.7,10,15 Weakness and collapse can also occur.14 Diarrhea and vomiting may result from intestinal hypermotility or ileus or may be secondary to underlying intestinal disease. Physical examination findings include dull mentation, fever (up to 108°F or 42°C), dehydration, mucosal pallor, abdominal pain, thin body condition, and/or abdominal enlargement and a palpable fluid wave due to ascites.7,10,14 Tachypnea or tachycardia may be present as a result of abdominal pain. However, some affected animals, and especially cats, show none of these signs. More than one third of cats lack any evidence of abdominal pain.10,15 Signs of septic shock may be present, such as tachycardia, tachypnea, weak pulses, and injected mucous membranes in dogs, or hypothermia and bradycardia in cats (see Chapter 86).10,15 Diagnosis of bacterial peritonitis is based on abdominal imaging findings, cytologic examination of peritoneal fluid, and aerobic and anaerobic bacterial culture of the fluid. Complete Blood Count and Serum Biochemical Tests Frequent findings on the CBC in dogs and cats with bacterial peritonitis are leukocytosis due to neutrophilia, a mild to severe bandemia, and monocytosis.7,10,12 Neutrophil toxicity is often present. Some animals are leukopenic (as low as 270 cells/µL in cats and 1000 cells/µL in dogs).7,10,15 Anemia of inflammatory disease may be identified. Thrombocytopenia may be present in animals with septic shock and DIC. The serum biochemistry panel often shows electrolyte and acid-base abnormalities and/or hypoalbuminemia due to inflammation or third-space losses. Mild to moderate increases in liver enzyme activities, hypoglycemia, or hyperglycemia may be detected; hyperbilirubinemia can be present in cats.7,10 Assessment of acid-base status in both dogs and cats most often reveals acidemia; hyperlactatemia is present in at least 50% of affected animals.7,10,15 Abdominal radiographs in dogs and cats with peritonitis may show a focal or diffuse loss of abdominal detail due to variable amounts of peritoneal effusion. In some animals, pneumoperitoneum is identified secondary to rupture of an abdominal viscus or penetrating abdominal trauma. Evidence of gastrointestinal obstruction or intestinal mass lesions may also be present.10,14 In tachypneic animals, thoracic radiographs should be considered to determine whether a pulmonary problem (such as acute respiratory distress syndrome or pulmonary thromboembolism) is contributing to respiratory distress. Findings on abdominal ultrasound examination include focal or diffuse hyperechogenicity of the mesentery and the presence of ascites fluid (Figure 88-1). The latter may have an echogenic appearance if it is an exudate. Evidence of underlying disease may be present, such as intestinal mass lesions or foreign bodies. The presence of pneumoperitoneum; dilated, air-filled bowel loops; or severe abdominal pain may interfere with complete ultrasound examination of some patients. CT examination is preferred to ultrasound for evaluation of human patients with bacterial peritonitis,7,8 but its usefulness for diagnosis and treatment of peritonitis in dogs and cats requires further investigation. FIGURE 88-1 Necropsy image from a 7-year-old terrier mix with severe bacterial peritonitis secondary to perforated intestinal lymphoma. The dog had acute onset of diarrhea and collapse before being brought to the veterinary clinic in cardiorespiratory arrest. On ultrasound examination there was a large volume of echogenic peritoneal effusion and the mesentery was severely hyperechoic. The stomach, small intestine, and colon were fluid distended, hypomotile, and had thickened, corrugated walls with diminished blood flow. At necropsy, the omentum was discolored dark red to purple, and a segment of jejunum had an intramural mass with a central depression that communicated with the intestinal lumen. (Courtesy University of California, Davis, Veterinary Anatomic Pathology Service.) Specimens for cytologic examination from animals with peritonitis may be collected by blind or ultrasound-guided abdominocentesis or diagnostic peritoneal lavage (DPL). With the increased availability and sensitivity of ultrasound to detect and guide collection of small quantities of undiluted intra-abdominal fluid, DPL is now uncommonly performed in the author’s practice. Peritoneal fluid from dogs and cats with bacterial peritonitis is typically a modified transudate or exudate, although fluid from dogs with primary peritonitis may be more likely to be a modified transudate or transudate.7 Fluid analysis from most animals with peritonitis reveals a high protein concentration and an increased erythrocyte and total nucleated cell count (usually >500 cells/µL and up to 160,000 cells/µL). There is typically a predominance of neutrophils that may have a degenerate appearance, and foreign material and/or intracellular and extracellular bacteria may be seen. The presence of intracellular bacteria is generally considered diagnostic for bacterial peritonitis. Because an absence of bacteria does not rule out bacterial peritonitis, submission of fluid for culture is essential.10,14 A blood-to-fluid glucose difference greater than 20 mg/dL was 100% sensitive and 100% specific for a diagnosis of bacterial peritonitis in one study of dogs and cats.18 Treatment of peritonitis involves a combination of antibiotic treatment, supportive care, and surgery. The goal of surgery is to identify and correct the source of leakage and to lavage and drain the abdomen. The latter is critical for effective antibiotic penetration. Cytologic evidence of inflammation with intracellular bacteria in peritoneal fluid specimens and/or the identification of free air within the abdomen (in the absence of a history of surgery or abdominocentesis) are indications for surgical exploration. Parenteral antimicrobial drug treatment should be initiated as soon as possible after diagnosis of peritonitis, because delayed antimicrobial drug treatment in severe sepsis and septic shock may increase mortality (see Chapter 86). The initial choice of antimicrobial drugs in dogs and cats with bacterial peritonitis should include a broad-spectrum combination of antimicrobials with activity against both obligate anaerobes and facultative anaerobes (especially E. coli and Enterococcus) (Table 88-3). Subsequently, treatment should be adjusted on the basis of cytology and culture and susceptibility results. Because obligate anaerobes may not grow reliably in the laboratory and are commonly involved, continued use of an antibiotic that provides activity against anaerobes is recommended even when anaerobic cultures are negative. TABLE 88-3 Suggested Empiric Antimicrobial Drug Choices for IV Use in Dogs and Cats with Bacterial Peritonitis Pending the Results of Culture and Susceptibility∗ Dosages for IV administration (normal renal function). See Chapter 8 for precautions. Amikacin 15-30 mg/kg q24h (dogs), 10-14 mg/kg q24h (cats) Ampicillin-sulbactam, 20 mg/kg q6-8h (dose based on ampicillin component) Gentamicin sulfate, 14 mg/kg q24h (dogs), 8 mg/kg q24h (cats) Ciprofloxacin hydrochloride, 10 mg/kg q24h Enrofloxacin, 5-20 mg/kg q24h (dogs); avoid in cats and never exceed 5 mg/kg, see important cautionary notes in Chapter 8 Imipenem-cilastatin, 5 mg/kg q6h (dilute in 100 mL sterile saline and give slowly over 30 min) Piperacillin-tazobactam, 40 mg/kg q6h ∗If appropriate, reduce spectrum once the results of culture and susceptibility are available. Careful surgical exploration for a site of bacterial leakage is indicated after initial stabilization with fluids, vasopressors, and antimicrobial drug treatment. The underlying cause must be surgically corrected (e.g., intestinal resection and anastomosis), and the peritoneum should be debrided and lavaged thoroughly with warm isotonic saline. The need for subsequent drainage is controversial. Drainage is generally selected when debridement and lavage cannot adequately reduce contamination. Methods of drainage include primary closure with closed suction drains, open peritoneal drainage, and, most recently, vacuum-assisted peritoneal drainage.10,12,17,20–22 In retrospective studies, no significant differences in survival have been identified among methods, but prospective studies are required. Complications of drainage are ascending nosocomial infection, obstruction of closed suction drains by omentum, and hypoproteinemia. Evisceration and bowel desiccation have the potential to occur with open peritoneal drainage, and fluid production cannot be quantified. Dogs and cats that undergo open drainage are also more likely to require plasma or blood transfusions and may have longer hospitalization times.17 Frequent sedation or anesthesia for bandage changes is also required. Supportive care for cats and dogs with bacterial peritonitis generally includes intensive crystalloid and colloid fluid therapy, administration of vasopressors, and enteral or parenteral nutritional support. Blood transfusions may also be required. Dogs that received early nutritional support had significantly shorter hospitalization times than those for which nutritional support was delayed.13 Survival rates of 32% to 67% have been reported in dogs with secondary peritonitis.7,9,11,12,16,21,22 Survival rates of 44% to 70% after surgery have been reported for cats.7,10,15 Typically hospitalization is required for 3 to 16 days (median around 5 to 6 days). When death occurs, it usually results from septic shock, DIC, or multiple organ dysfunction. Negative prognostic indicators identified in dogs with bacterial peritonitis include a diagnosis of primary as opposed to secondary peritonitis7; preoperative anemia, leukocytosis, or hypoproteinemia16; and postoperative administration of glucocorticoids.16 Low preoperative systolic blood pressure was a negative prognostic factor in a study that included both dogs and cats.14 Low preoperative median serum ALT activity (56 U/L for surviving cats compared with 179 U/L for non-survivors) was the only positive prognostic indicator in one study of cats with peritonitis.10 Age, preoperative heart rate, rectal temperature, the results of other laboratory parameters, or the presence of polymicrobial infection did not affect survival. In another study, a high plasma lactate concentration was a negative prognostic indicator in cats with bacterial peritonitis.15
Intra-abdominal Infections
Infectious Gastroenteritis
Organism Type
Dogs
Cats
Viruses
Canine parvovirus
Canine enteric coronavirus
Canine distemper virus
Rotaviruses
Astroviruses
Adenoviruses
Caliciviruses
Feline panleukopenia virus
Feline coronavirus
Feline calicivirus
FeLV
FIV
Rotaviruses
Astroviruses
Torovirus-like agent
Reoviruses
Bacteria
Salmonella spp.
Clostridium perfringens
Clostridium difficile
Campylobacter spp.
Helicobacter spp.
Escherichia coli
Klebsiella pneumoniae1
Enterococcus spp.2
Yersinia enterocolitica
Brachyspira pilosicoli3
Mycobacterium spp. (e.g., M. avium)
Leptospira spp.
Neorickettsia helminthoeca
Salmonella spp.
C. perfringens
C. difficile
Campylobacter spp.
Helicobacter spp.
Escherichia coli
Enterococcus spp.4
Y. enterocolitica
Anaerobiospirillum5
Mycobacterium spp. (e.g., M. bovis)
Protozoa
Giardia spp.
Entamoeba histolytica
Balantidium coli
Isospora spp.
Hammondia heydorni
Cryptosporidium spp.
Leishmania spp.
Tritrichomonas foetus
Giardia spp.
E. histolytica
Isospora spp.
Cryptosporidium spp.
Tritrichomonas foetus
Fungi
Histoplasma capsulatum
Cryptococcus neoformans
Blastomyces dermatitis
Candida albicans
Aspergillus spp.
Zygomycetes
Pythium insidiosum
Prototheca spp.
Histoplasma capsulatum
Candida albicans
Other parasites
Toxocara canis and Toxascaris leonina (roundworms)
Ancylostoma and Uncinaria spp. (hookworms)
Trichuris vulpis (whipworms)
Tapeworms (diphyllobothriidean)
Toxocara cati and Toxascaris leonina (roundworms)
Ancylostoma and Uncinaria spp. (hookworms)
Tapeworms (diphyllobothriidean)
Bacterial Peritonitis
Organ System Affected
Disease Process
Gastrointestinal system
Gastrointestinal surgical site dehiscence (e.g., resection and anastomosis)
Penetrating abdominal trauma
Nonsteroidal or steroidal anti-inflammatory drug toxicity
Foreign bodies
Gastrointestinal neoplasia
Eosinophilic gastroenteritis
Torsion
Intussusception
Genitourinary tract
Ruptured pyometra
Surgical site dehiscence (e.g., ovariohysterectomy)
Ruptured prostatic abscess
Necrotizing bacterial cystitis
Hepatobiliary system
Gallbladder rupture (especially animals with necrotizing bacterial cholecystitis)
Hepatic abscess rupture
Other abdominal organs
Abscess rupture
Umbilicus
Ascending infection in neonates
Clinical Features
Diagnosis
Laboratory Abnormalities
Diagnostic Imaging
Sonographic Findings
Microbiologic Tests
Treatment and Prognosis
Antimicrobial Treatment
Antimicrobial Drug
Spectrum
Comments
Ampicillin-sulbactam and a fluoroquinolone (e.g., enrofloxacin, marbofloxacin)
Activity against gram-negative bacteria, some methicillin-resistant staphylococci, streptococci, enterococci, and most anaerobes
Replace the fluoroquinolone with an aminoglycoside (amikacin or gentamicin) if the regional prevalence of fluoroquinolone resistance is high.
Metronidazole and a fluoroquinolone
Activity against susceptible gram-negative and gram-positive bacteria and anaerobes
Replace the fluoroquinolone with an aminoglycoside (amikacin or gentamicin) or a third-generation cephalosporine if the regional prevalence of fluoroquinolone resistance is high.
Ticarcillin–clavulanic acid
Activity against susceptible gram-positive and gram-negative aerobes (including Pseudomonas aeruginosa) and some anaerobes
Not active against methicillin-resistant staphylococci
Carbapenem (meropenem or imipenem-cilastatin)
Activity against susceptible gram-positive and gram-negative aerobes and anaerobes
Not active against methicillin-resistant staphylococci. Reserve use for when multidrug-resistant gram-negative bacterial infection is suspected.
Piperacillin-tazobactam
Activity against susceptible gram-positive and gram-negative aerobes, including Pseudomonas aeruginosa, and some anaerobes
Not active against methicillin-resistant staphylococci. Reserve use for when multidrug-resistant gram-negative bacterial infection is suspected.
Surgical Treatment
Supportive Care
Prognosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


