CHAPTER 17 The integumentary system
The skin is the largest organ of the mammalian body. It protects the body against dehydration, injury and infection and, moreover, it is constantly renewable.
EPIDERMIS
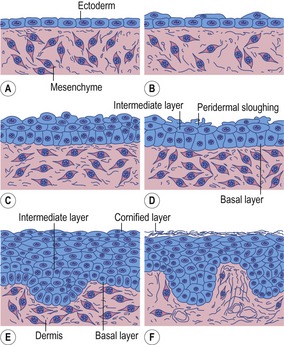
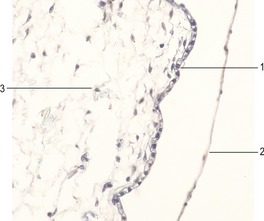
The epidermis originates from the ectodermal cells covering the embryo after completion of neurulation (Figs 17-1, 17-2). This is a single-layered epithelium with its apical plasma membrane, facing the amniotic cavity, possessing occasional microvilli. The cells of this single-layered surface ectoderm start to proliferate in the early embryo and form a second protective layer – the periderm or covering layer (Fig. 17-3). The periderm is a temporary covering that is shed once the inner layers differentiate to form a true epidermis. With further proliferation of the cells of the basal layer, a third, intermediate layer is formed. Finally, the epidermis acquires its definitive arrangement and four layers can be distinguished: a) the basal layer or germinative layer (stratum basale); (b) the spinous layer (stratum spinosum); (c) the granular layer (stratum granulosum), and (d) the cornified layer (stratum corneum).
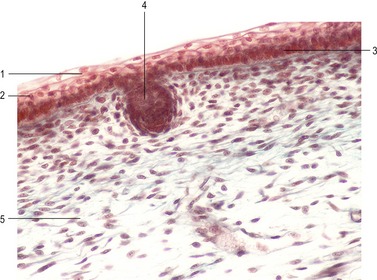
Fig. 17-3: Epidermis of a cat embryo with a CRL of 46 mm. 1: Periderm; 2: Intermediate layer of the epidermis; 3: Basal layer of the epidermis; 4: Epidermal downgrowth; 5: Mesenchyme.
Cells in the basal layer sitting on the basal lamina will become the germinative layer, a zone destined to give rise to the stratified epithelium of the epidermis. The basal layer contains the epidermal stem cells that divide mitotically to continuously replace the upper layers of the epidermis, which cornify and eventually get sloughed off. The stem cells of the epidermis divide asymmetrically: the daughter cell that stays attached to the basal lamina remains a stem cell, whereas the cell that leaves the basal layer and migrates to the surface starts to differentiate. The latter cells produce keratins characteristic of skin and arrange them into dense intermediate filaments. The differentiated epidermal cells are called keratinocytes. They are bound tightly together by desmosomes and produce a water-impermeable seal of lipid and protein that minimizes dehydration of the body (Fig. 17-4).
Continuous cell production in the basal layer generates cells that push older cells to the surface of the epidermis. The movement of epidermal cells from the basal layer is preceded by a loss of adhesiveness to basal membrane molecules, like fibronectin, laminin and collagen types I and IV. These cellular changes can be explained by the loss of several membrane proteins (integrins) from the plasma membrane of basal epidermal cells that mediate the attachment of these cells to basal lamina components. Cells of the spinous layer produce prominent bundles of keratin filaments, which converge on desmosomes, binding the cells to each other. During their migration, synthesis of differentiated products, like keratins, ceases in the cells. Keratohyalin granules begin to appear in the cytoplasm of the outer, postmitotic cells of the spinous layer and become prominent components in the next layer, the granular layer. The cells become flattened and their nuclei are pushed to one edge of the cells as they move into the outermost cornified layer. There, the cells eventually lose their nuclei and resemble more or less flattened sacs packed with keratin filaments and interconnected by the histidine-rich protein filaggrin. The keratinocytes of the cornified layer are continuously shed. The journey from the basal layer to the sloughed cells takes about 2 weeks in mice, 3 weeks in pigs, and 4 weeks in humans.
Several other cell types, which are generally termed epidermal non-keratinocytes, can be identified in the developing epidermis. Neural crest cells migrate into the dermis and differentiate into melanoblasts. They also invade the basal layer of the epidermis. Melanoblasts produce melanosomes containing the brown pigment melanin, which is made by oxidation of L-tyrosine in the presence of the enzyme tyrosinase. Melanosomes are transported to neighbouring keratinocytes and commonly make up the different body colour patterns observed in mammals. Merkel cells are intraepidermal mechanoreceptors associated with free nerve terminals. They were once regarded as specialized keratinocytes but new data support the idea that Merkel cells are of neural crest cell origin. Late in prenatal development, the epidermis is invaded by cells of another type, the Langerhans cells, which arise from precursors in the bone marrow. These cells are regarded as peripheral components of the immune system and are involved in the presentation of antigens. They cooperate with T-lymphocytes in the skin to initiate cell-mediated responses to foreign antigens.
DERMIS (CORIUM)
The future dermis initially forms from loosely aggregated mesenchymal cells that are tightly interconnected by focal tight junctions on their cellular processes. These mesenchymal cells secrete an intercellular matrix rich in hyaluronic acid and glycogen. Later they differentiate into fibroblasts, which form increasing amounts of collagen (types I and III) and elastic fibres (Fig. 17-1).
EPIDERMAL APPENDAGES
Hair
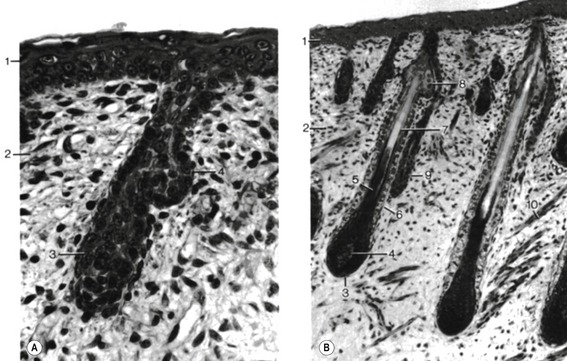
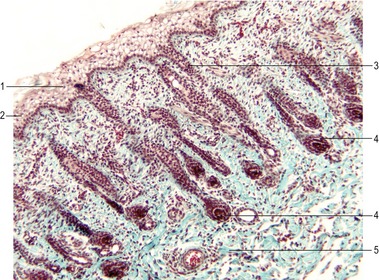
Hairs appear as solid proliferations from the basal layer of the epidermis that penetrates the underlying dermis. The basal epidermal cells divide, elongate and penetrate the dermis at an oblique angle. At their terminal ends, these hair buds (hair germs, hair pegs) invaginate. The invaginations are rapidly filled with mesenchymal cells and form the dermal papillae which contain blood vessels and nerve endings. Under the continuing influence of the dermal papilla, the cells of the epidermal downgrowth continue to divide and form an early hair bud. Soon the cells in the centre of the hair bud become spindle-shaped and keratinized. They form the hair shaft, while the peripheral cells remain more cuboidal, giving rise to the epithelial hair sheath that later develops into the internal and external root sheath. Around the epidermal hair sheath, the surrounding mesenchyme forms a dermal root sheath (Fig. 17-5).
The spacing of the primary hair follicles involves the influence of several growth and transcription factors. FGF-5, produced by the surrounding mesenchyme, has a stimulatory effect on the epidermal downgrowth of the hair buds. Counteracting this is an inhibitory influence of BMP-2 and BMP-4, which may regulate the spacing between adjacent hair buds. Soon after its formation, the hair bud begins to express Sonic hedgehog (Shh), which stimulates cell proliferation and growth of the hair follicles.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree