Chapter 27 Infrequently Encountered Gram-Negative Rods
BARTONELLA SPECIES
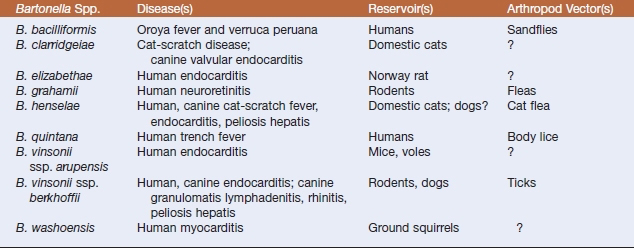
Bartonella is the only genus of the family Bartonellaceae. These aerobic, slowly growing, facultatively intracellular, gram-negative organisms are placed in the α-division of the Proteobacteria based on their 16S rDNA sequences. The genus includes bacteria formerly classified in the genera Grahamella and Rochalimaea; some of the 18 species are presented in Table 27-1. All are fastidious, argyrophilic, hemotrophic, and highly adapted to a mammalian reservoir host. They cause a variety of clinical syndromes in both immunocompetent and immunocompromised humans and animals. Diseases caused by bartonellae are usually vector-borne, with transmission by arthropods (lice, fleas, ticks, biting flies, mites, chiggers). Transmission as a result of blood transfusions has also been documented.
Bartonella henselae is the etiologic agent of cat-scratch fever, which is also transmitted by cat fleas. Manifestations of human and canine disease may include prolonged fever, meningitis, bacillary angiomatosis, and peliosis hepatis. Bacillary angiomatosis is a neovascular proliferative disorder that may involve skin, regional lymph nodes, liver, spleen, brain, bones, bowel, and lungs. Peliosis hepatis–affected tissue contains numerous blood-filled partially endothelial cell-lined cystic structures. Chronically bacteremic cats are the reservoir. Bartonella henselae may also be an important emerging pathogen of dogs. Polyarthritis, weight loss, seizures, epistaxis, and endocarditis may result from inoculation by way of cat scratches.
Lymph node biopsies or aspirates, affected portions of liver or spleen, and blood are the specimens of choice. Efficiency of blood culture is increased by lysis of erythrocytes. Blood collected in EDTA is held at −70° C for 24 hours before inoculation onto plated isolation media. Colonies of Bartonella spp. appear in 10 days or more on solid media, and freshly prepared heart infusion agar with 5% to 10% horse or rabbit blood supports better growth than chocolate or sheep blood agar. Primary culture plates should be sealed against dehydration after 24 hours’ incubation and incubated for up to 6 weeks before being discarded as negative. Growth is optimal at 37° C in 5% to 7% CO2 for all species except B. bacilliformis, which has an optimal growth temperature of 25° to 28° C and grows best without supplemental CO2.
Bartonella colonies usually exhibit two morphotypes (Figure 27-1). One is irregular, whitish, raised, rough, and dry, and may be cauliflower-like in appearance. The other type is small, tan, circular, moist, entire, and adherent to the agar. Gram stains of the colonies reveal tiny, slightly curved, faintly staining, gram-negative bacilli. Most Bartonella spp. are oxidase, catalase, urease, and nitrate reductase negative, and carbohydrates are not utilized. Genus and species iden tification is best accomplished by molecular methods.
CDC GROUP EF-4A
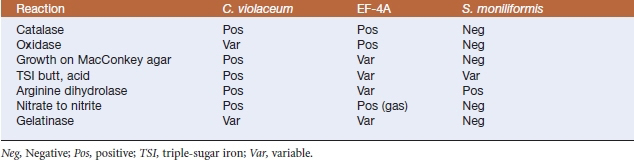
Diagnosis is by culture of affected tissues. After 48 hours of aerobic incubation on blood agar plates, colonies are 1 mm in diameter, nonhemolytic, convex, round, opaque, and nonpigmented to yellowish with a distinctive popcornlike odor. Isolates are catalase and oxidase positive, and growth is sometimes seen on MacConkey agar. Triple-sugar iron (TSI) agar (butt and slant) have an initial weak acid reaction. Other distinguishing features include nitrate reductase, gelatinase, and arginine dihydrolase activities, acid production from glucose, and inability to produce urease or indole (Table 27-2).
TABLE 27-2 Selected Biochemical Reactions of Chromobacterium violaceum, EF-4A, and Streptobacillus moniliformis
These organisms are reported to be susceptible in vitro to ampicillin, aminoglycosides, sulfa drugs, and tetracyclines. Resistance to narrow-spectrum cephalosporins, trimethoprim-sulfamethoxazole, penicillin, and erythromycin has been reported.
THE GENUS CHROMOBACTERIUM
Diagnosis is based on bacterial isolation and identification. Colonies are 0.5 to 1.5 mm in diameter, smooth, and convex with entire edges and β-hemolysis. Growth may occur at 25° and 42° C. A faint violet tint generally develops after 24 hours’ incubation on blood agar, and the color becomes deeper in subsequent days. Colonies on MacConkey agar are colorless. Most isolates produce oxidase and catalase, but are negative for Voges-Proskauer reaction and do not hydrolyze esculin. There is an acid reaction on TSI slants, but H2S is not produced. Arginine dihydrolase is produced. Oxidase-positive nonpigmented strains can be differentiated from Aeromonas or Vibrio spp. by the ability of the former to grow in nutrient broth without salt and to ferment glucose, mannitol, and maltose, and by their lack of lysine and ornithine decarboxylase activities. Oxidase-negative strains can be mistaken for enteric bacilli (see Table 27-2).