Infertility and Subfertility in the Male
Assessment of Male Fertility
History and Initial Clinical Evaluation
The male alpaca or llama reaches sexual maturity at age 4 years, with a wide range of variability. Puberty is defined as the ability of a male to exhibit breeding behavior and to have sufficient spermatogenic development to get a female pregnant. Young male camelids may demonstrate mounting behavior as young as age 6 months, although preputial attachments will prohibit full erection and intromission.1 In pasture management systems, crias should be segregated on the basis of sex, especially after weaning, to minimize potential unplanned early matings.
The examination is tailored to the age and maturity of the male. Very young males will be evaluated on physical parameters only. A general physical examination is performed, noting any musculoskeletal abnormalities that may impair breeding behavior in the future. The external genitalia are examined for any congenital defects. The scrotum is evaluated for two descended testes, which should be of the same size, with up to 15% variation.2 Often the testes are offset slightly in the dorsoventral orientation. The testes are measured using calipers or with ultrasonography and the length and width recorded in millimeters.3,4 Ultrasonographic examination of the testes is performed using a linear 7.5 megahertz (MHz) transducer. The parenchyma is evaluated in the longitudinal (sagittal) and transverse orientations (Figure 19-1). Any abnormalities, including the presence of rete testis cysts, should be noted. Any cysts should be measured at the widest diameter. The presence of small cysts should not exclude the male from being used as a sire, but regularly scheduled evaluation (every 6 to 12 months) with ultrasonography helps identify any changes.5 The testicular dimensions in a young male continue to increase as the animal attains sexual maturity. The accessory sex organs are not routinely examined. However, the prostate and bulbourethral glands are easily assessed for size and general appearance by using ultrasonography. The bulbourethral gland is imaged just cranial to the anal sphincter by slightly rotating the transducer from middle at a 40-degree angle (Figure 19-2, A). The gland is homogeneous until stimulated. The examination continues along the midline to image the pelvic portion of the penis (see Figure 19-2, B). The prostate is wrapped around the cranial border of the pelvic penis and is recognized as an oblong bilobed homogeneous body (see Figure 19-2, C). Determination of the depth of the prostate is important for positioning the electrode of the electroejaculator during semen collection with this technique.
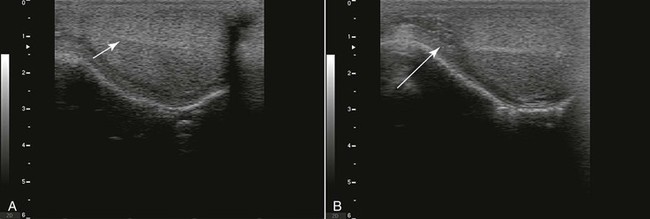
Figure 19-1 Normal Ultrasonography of a Llama Testis in Sagittal View.
A, Note the homogeneous testicular parenchyma and the hyperechoic central line corresponding to the rete testis (arrow). B, Normal ultrasonogram of the head of the epididymis area (arrow).
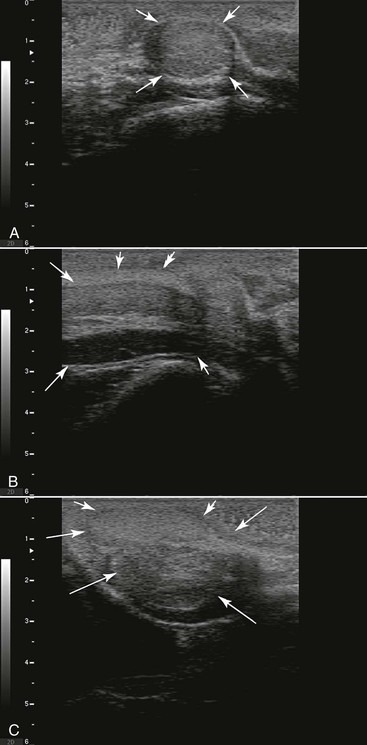
Figure 19-2 Normal Ultrasonography of the Accessory Sex Glands in Camelids.
A, Bulbourethral gland is round, almost spherical; it is homogeneous when not stimulated. An echolucent zone is seen at the cranial edge. B, The arrow delineates the pelvic portion of the penis. C, Prostate delineated by arrows.
All parameters should be recorded, and males should be examined at least yearly. Males should also be presented for evaluation if the conception rate of bred females drops acutely or if any physical abnormalities of the male are noted. Additionally, any signs of systemic illness or fever must be promptly addressed to minimize any potential effects on spermatogenesis. Lastly, males should be maintained on proper nutrition with adequate trace mineral supplementation.
Testicular Biopsy
Testicular biopsy in the male is not a commonly employed diagnostic tool but may be invaluable in cases of azoospermia or oligozoospermia when no other causes are identified. Additionally, biopsy may be diagnostic for neoplastic or degenerative changes and is warranted in cases of testicular asymmetry or abnormal testicular ultrasonography. In cases in which testicular hematoma or orchitis is suspected, testicular biopsy is contraindicated.6
Several methods of testicular biopsy have been used in alpacas and llamas, with varying results and complications. These methods include a wedge biopsy, Tru-cut needle biopsy, core self-firing biopsy, or fine-needle aspiration.6
Wedge biopsy, also called incisional or open biopsy, requires the use of general anesthesia. The scrotum is aseptically prepared, and a small incision is made over the selected testicle through the skin, parietal vaginal tunic, and the exposed tunica albuginea, taking care to avoid vascularized areas. The testicular tissue will protrude through this incision in the tunica albuginea, and a scalpel blade is used to remove this section. The tunica albuginea, parietal vaginal tunic, and scrotal skin are closed with absorbable sutures. This type of biopsy provides a good sample of tissue for evaluation of the architecture of the seminiferous tubule and spermatogenesis, but its use has diminished because it is unsafe and has a very high rate of complications, including hematoma, hemorrhage, formation of testicular adhesions, inflammation, infection, and immune-mediated reactions. Additionally, degeneration of the germinal epithelium and seminiferous tubules may occur, and a transient decrease in spermatogenesis and spermatozoa output may be seen for several months.6
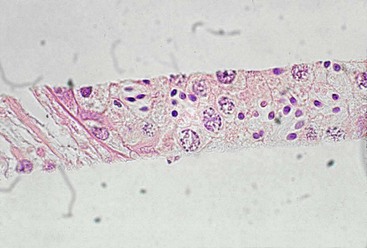
A core self-firing biopsy instrument (Bard Biopsy Systems, AZ) is the best choice for testicular biopsy (Figure 19-3). The self-firing mechanism decreases the amount of testicular damage inflicted during the procedure, with hematoma formation as the primary complication. As with the Tru-cut biopsy, the scrotum is aseptically prepared, the scrotal skin incised with a scalpel, and the biopsy sample obtained. The tip of the instrument (14- to 18-gauge, depending on the male) is placed against the tunica albuginea and the instrument fired. The scrotum is left open. Samples are adequate for histologic evaluation (Figure 19-4). In our practice, males undergoing this procedure are given a single dose of long-acting ceftiofur preparation and flunixin meglumine for 2 days. No complications have been observed in our practice.
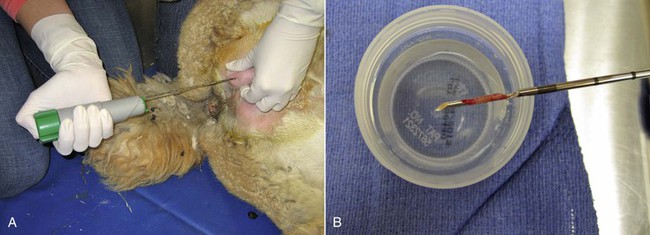
Figure 19-3 Testicular Biopsy Technique Using the Bard Biopty Instrument.
A, After surgical preparation, a small incision is made through the scrotal skin and vaginal tunic, the needle is placed against the albuginea, and the spring loaded instrument is triggered. B, The sample is a sliver of testicular parenchyma of about 3 × 15 mm and is sufficient for histologic evaluation.
The safest method of testicular biopsy is also the least diagnostic.3 Fine-needle aspiration may be performed without the use of general anesthesia. A 20-gauge 1-inch needle is fitted to a 12-mL syringe and introduced into the testicular parenchyma, taking care to avoid the epididymis. Tissue is aspirated, redirecting the needle several times. The cells are then injected onto slides, which are stained with DiffQuick or Wright-Giemsa stain. A representative sample of cells may be obtained; however, the cellular architecture of the seminiferous tubules cannot be evaluated. Interpretation of the sample, therefore, is based on the amount of cellular material and presence of specific cell types (spermatogenic, Sertoli cells, Leydig cells) and is scored from 0 to 3 (Box 19-1; Figure 19-5). Spermatogenic cells are scored from 1 to 5 (see Box 19-1). Twenty-five percent of aspirated samples are nondiagnostic based on the cellular population obtained. Fine-needle aspiration has a low rate of complications, but the male should still be monitored for signs of inflammation or infection.
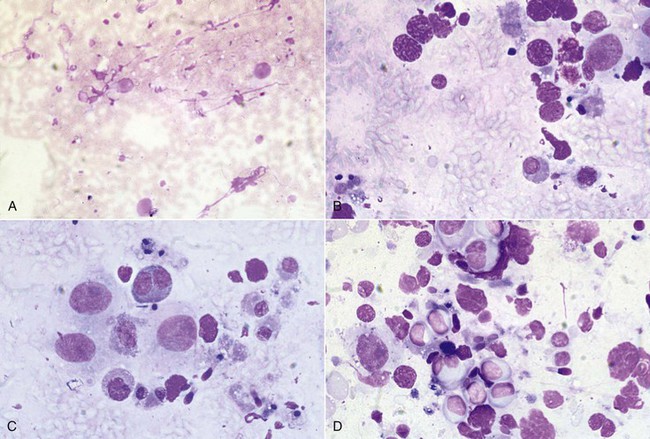
Figure 19-5 Testicular Fine-Needle Aspiration Evaluation.
A, Poor cellularity and absence of spermatogenesis. B, Spermatogonia and spermatocytes, but no spermatids. C, Evidence of spermatogenic activity and presence of elongated spermatids. D, Active spermatogenesis; note the increased cellularity and presence of differentiated spermatozoa. (Reprinted with permission, Tibary A and A Anouassi A, Theriogenology in Camelidae, second edition, Actes Editions, Institut Agronomique et Veterinarie Hassan II, Rabat, Morocco.)
Poor Libido
Poor libido in a male is characterized by the animal not demonstrating typical breeding behaviors or a sharp decline in normal behaviors. This may be seen as declining interest in the female and decreased breeding times and may be related to physical or systemically debilitating conditions. Poor libido should not be confused with sexual immaturity in young males or apparent shyness, especially if post-mating collection of an ejaculate is planned in a hospital setting.
Poor libido may have an endocrinologic basis, and serum testosterone may be assessed. Supplementation with testosterone is contraindicated in cases of low serum levels because of the negative effects on spermatogenesis and downregulation of the hypothalamo–pituitary–gonadal axis. Poor libido may also be caused by abnormalities outside the reproductive tract. Systemic illness may play a role, and a complete blood cell count and serum biochemistry panel may demonstrate signs of inflammation or abnormalities in other organ systems. Rectal temperature may demonstrate a febrile condition. Megaesophagus has been demonstrated to be linked to poor libido, as the affected males may experience gastric reflux during intromission, which would cause discomfort.7,8 High ambient temperatures may also play a role.
Treatment of poor libido is tailored to any identified abnormalities. Systemic illness should be addressed appropriately. Fever may be treated with systemic antiinflammatory medications and specific therapy for the inciting cause. Treatment for megaesophagus relies heavily on attempts to distinguish the cause. Megaesophagus may be congenital or acquired; in acquired cases, only organophosphate toxicity has been demonstrated to have a causal effect. Other suspected causes include endocrine disorders, parasitic infestations, infectious diseases, nutritional derangements, trauma (iatrogenic from venipuncture, which affects the branches of the vagal nerve), immune-mediated or neoplastic disorders, all of various etiologies.7 Treatment is tailored to the suspected inciting cause, with highly variable success rates. High ambient temperatures may be addressed by placing males in shaded areas with fans or water misters as well as shearing of the whole body, including the tail. Males that are inexperienced or shy may benefit from being exposed, over a fence-line, to a proven male actively mating with a receptive female. Often witnessing the mating behavior is enough to stimulate the male at that time to engage in the same manner with a receptive female.
Erection Failure
The prepuce and the penis should be thoroughly evaluated. Often this is not possible without heavy sedation or general anesthesia of the male. Any physical abnormalities should be identified. Physical problems of the reproductive tract are addressed later in this chapter; those which may interfere with erection include preputial disorders (edema, laceration, obstruction, prolapse) or penile disorders (trauma, adhesions, congenital disorders, urolithiasis, hair ring). Refer to Table 19-1 for a complete list of documented reproductive diseases of male alpacas and llamas.
TABLE 19-1
Documented Diseases of the Male Reproductive Tract of Alpacas and Llamas
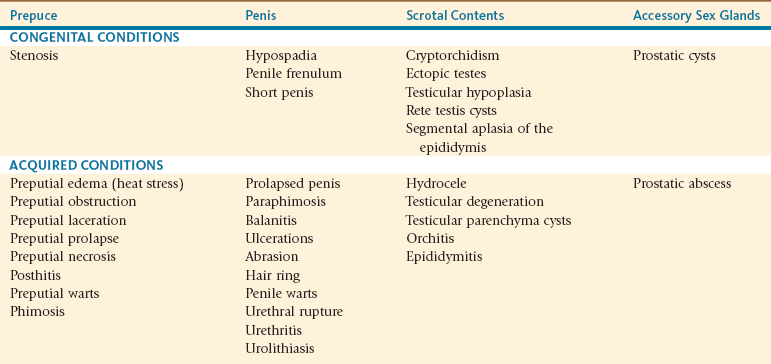
From Tibary A, Pugh D: Infertility in the male lamoid. In Proceedings Society for Theriogenology and American College of Theriogenologists Annual Conference, Columbus, OH, September 17-20, 2003 (pp 313-322).
In the absence of a physical cause of erection failure, neurologic causes must be explored. The neurologic damage may occur anywhere from various levels of the spinal cord to the penis itself. A neurologic examination should be performed and include watching the animal walk on flat and sloped surfaces and maneuver obstacles and for any proprioceptive or reflex deficits, especially in the hindlimbs. Neurologic disease from infection with Paraphostrongylus tenuis (meningeal worm) has been seen to cause erection failure in two male camelids.1
Ejaculation Failure
In the absence of physical causes, ejaculation failure is likely a neurologic problem. Unlike in other species in which the ejaculatory event is the culmination of the mating act and the ejaculatory threshold must be crossed to achieve ejaculation, alpacas and llamas ejaculate throughout the mating act, emitting a small volume several times per minute.9 The ejaculate is not fractionated.10
In the equine, ejaculation may be induced pharmacologically to obtain semen for breeding or cryopreservation. One research group evaluated the efficacy of pharmacologically induced ejaculation ex copula in male llamas using intravenous imipramine followed by intravenous xylazine. Results showed that only 30% of collected samples contained spermatozoa.11 In male alpacas or llamas that experience ejaculatory disorders, this may be a potential means to obtain semen for artificial insemination. However, ex copula induction of ejaculation has not been successful in our practice, and this technique needs further evaluation.
Azoospermia and Oligozoospermia
Males that experience azoospermia or oligozoospermia may be presented to the veterinarian for evaluation following a period of breeding in which the conception rate was greatly decreased or absent compared with previous conception rates with the same male. In cases of no previous history or poor breeding records, the time of onset of the condition and the etiology are hard to determine. Likewise, a male that had been used sporadically for breeding and then submitted for several matings per day may experience lower pregnancy rates in bred females, azoospermia, or both.12,13
A complete history of the male, including age, medical history, previous examination results or reports, vaccination and deworming dates, travel and show history, should be taken. Complete breeding records, including the number and length of matings, fertility of bred females, and pregnancy rates as well as pregnancy diagnosis method used, should be obtained from the owners. Additionally, any details regarding the management of the animal should also be provided to the veterinarian.2
Trace Mineral Deficiency
Trace mineral imbalance of copper, zinc, and selenium are associated with derangements in spermatogenesis and infertility.14–18
Serum analysis of trace mineral levels in animals that are presented for infertility may not be reflective of stored levels in tissue (mainly liver); however, animals whose serum demonstrates low trace mineral levels may benefit from supplementation, especially those living in areas of known soil deficiencies. The producer should be aware that correction of trace mineral imbalances may not restore fertility, especially in cases of azoospermia or heat shock, but may help improve the motility and morphology of produced spermatozoa and may improve other systemic health aspects of the animal or the herd.
Heat Stress
Fevers caused by infection may reach extremes of 106°F (A. Tibary, personal observations) and last for several days. This elevation in body temperature will elevate scrotal and testicular temperature to the point of arresting spermatogenesis (heat shock). Other causes of heat shock include high ambient temperatures, combined with lack of shearing or trailering for shows or other travel purposes. In some cases, heat shock results in temporary oligozoospermia or azoospermia, but in cases of high fever or high ambient temperature for longer than 3 days, often the effects on spermatogenesis are long lasting and potentially irreversible. It has been demonstrated that in times of high ambient temperatures, shorn animals will dissipate heat from the entire body, whereas unshorn animals will increase the respiratory rate to maintain temperature homeostasis.19 However, despite maintaining normal body temperature, animals subjected to high ambient temperatures had significant decreases in both ejaculate volume and spermatozoa concentration, with histologic changes in the seminiferous tubule architecture and derangements in spermatogenesis.19,20
Severely affected animals demonstrate the systemic effects of heat stress, including hyperthermia, depression, ataxia, weakness, dehydration, ketosis, hepatic lipidosis, and dyspnea.21 Serum electrolyte levels often demonstrate hypophosphatemia, hypocalcemia, hypomagnesemia, hyponatremia, hypochloridemia, and hypo- or hyperkalemia. Other derangements include hyperglycemia, elevations in alanine aminotransferase (AST) and creatine kinase (CK), anemia, and inflammatory leukogram. Prolonged derangements in untreated animals, especially in electrolyte levels, may lead to damage of the thermoregulatory center of the hypothalamus, which subsequently leads to multiorgan failure and death.21
The aim of the initial treatment is to cool and stabilize the animal with shearing, cold water, fans, and fluid therapy with added electrolytes to correct any imbalances identified in the serum biochemistry assay. Fluid rates should be monitored to prevent pulmonary edema. Dyspneic animals should be supplemented with intranasal oxygen. Additional treatments include correction of metabolic acidosis, antiinflammatory medications, antioxidants such as selenium and vitamin E, and broad-spectrum antibiotics to prevent secondary infection.21 Corticosteroids may be used in cases of severe inflammatory reactions but not in pregnant females because of risk of abortion. Scrotal and preputial edema should be monitored for resolution. Increased durations of increased scrotal temperature often lead to higher risks of spermatogenic arrest and subsequent infertility.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree