Infertility and Subfertility in the Female Camelid
Various congenital as well as acquired disorders of the reproductive tract in camelids have been described and may play an important role in reduced fertility.1 In many cases, diagnosis of the cause of infertility may require monitoring the female over at least one reproductive cycle (from follicular growth to mating and pregnancy diagnosis). The objectives would be to answer the following questions: What is the expertise of the breeder? Is the male fertile? Does the female have normal genitalia? Is the female ovulating? Judicious choice of examination techniques and interpretation allow reaching a diagnosis in an accurate and timely manner. The objective of the present chapter is to discuss the major presenting complaints with regard to camelid infertility as seen in practice, as well as the main reproductive disorders in the female camelid and the approach to diagnosis and treatment.
Common Presenting Complaints
The most common complaints related to infertility in female camelids can be categorized into repeat breeding, early pregnancy loss, abnormal behavior (continuous receptivity or rejection of the male), and visible abnormalities of the external genitalia. These categories of complaints represent 76%, 18%, 4%, and 2%, respectively, of all cases seen in our clinics.2–4
Repeat breeding syndrome is defined as the regular return into receptivity after a normal luteal phase. Therefore, all females that failed to become pregnant after two breeding cycles to a male with proven fertility should be included in this category. Repeat breeding syndrome may be caused by pathologic factors or breeding management errors. The pathologic factors involved in repeat breeding include all conditions that may affect fertilization or early embryo survival (Table 20-1).
TABLE 20-1
Documented Causes of Repeat Breeding in Female Camelids
| Category of Causes | Probable Causes | Likely Direct Cause |
| Fertilization failure | Management | Lack of expertise in breeding camelids, breeding in absence of mature follicle, incomplete breeding |
| Failure of ovulation | Incomplete breeding, lack of luteinizing hormone release, ovarian pathology, bad timing of mating, male abnormalities | |
| Failure of ejaculation | Male infertility, age of the male, male overuse | |
| Oviductal transport problem | Uterine tube pathology, ovarian bursa pathology | |
| Poor survival of semen | Uterine pathology, male infertility, semen quality (artificial insemination) | |
| Early embryo loss | Abnormal embryos | Cytogenetic errors, poor quality semen or ova |
| Abnormal uterine environment | Uterine infections, uterine fibrosis | |
| Hormonal factors | Corpus luteum dysfunction | |
| External factors | Problems related to management, nutrition, heat stress |
Early pregnancy loss is generally defined as a return to receptivity following a positive pregnancy diagnosis.3 Early pregnancy loss may be apparent as a return to receptivity after behavioral manifestation of luteal function (male rejection) or following a positive diagnosis of luteal activity through progesterone assay. In a strict definition, early pregnancy loss can only be ascertained if the female has been previously diagnosed as being pregnant by using ultrasonography.
Abnormal behavior may be categorized as complete lack of receptivity, continuous receptivity, or aberrant or male-like behavior. Lack of receptivity or persistent rejection of the male (spitting off) is often caused by elevated plasma progesterone levels. It is imperative to first rule out pregnancy before proceeding with more invasive or expensive diagnostic techniques. Elevated progesteronemia in absence of pregnancy is from persistent luteal function or presence of luteinized hemorrhagic follicles. Both pregnancy and the presence of luteal tissue can easily be confirmed by using ultrasonography. In rare cases, females may show a lack of receptivity because of absence of ovarian follicular activity (ovarian dysgenesis or hypoplasia). Lack of receptivity may be also elicited by painful copulation or abnormal socialization.
In general, a thorough clinical examination, as we described elsewhere in this text (see Chapter 17: Clinical Examination of the Female Reproductive Function), enables diagnosis of the majority of cases.
Repeat Breeding Syndrome
The approach for an accurate diagnosis of the cause of repeat breeding involves a complete history of the animal and the herd, clinical evaluation of the female, and laboratory findings to rule out any possible cause of infertility (Table 20-2).
Unfortunately, signs of estrus in camelids are not reliable and lack any significant correlation to follicular growth rate and follicular size, which are critical for the timing of breeding and subsequent induction of ovulation.5 Diagnosis of the cause of repeat breeding can only be achieved with a complete and systematic approach to the problem.1,4 In a normal breeding management system, a female is mated, spits off the male at 1 week, and becomes receptive again 12 to 15 days after mating if not pregnant. This typical behavior suggests that the female is ovulating normally but experiences a failure of fertilization or an embryonic loss before maternal recognition of pregnancy (before days 9 to 10 after mating).
Early Embryonic Death
Camelids require ovarian progesterone (presence of a functional corpus luteum) for maintenance of pregnancy; therefore, any alteration of the corpus luteum function either by disturbance of maternal recognition of pregnancy or compromise of the luteal activity (iatrogenic administration of luteolytic drug) will result in early pregnancy loss.5
Incidence of early embryonic death in llamas and alpacas may be as high as 57.8%.6 Most of the early embryonic loss occurs before day 45 of pregnancy, and it is suspected when the female becomes receptive again or fails to show signs of advanced pregnancy. Some of the possible etiologies of embryonic death in camelids include genetic or environmental factors (heat stress), corpus luteum dysfunction, twining, and uterine pathology such as infection or fibrosis. Also, deficiencies in vitamins A, E, or selenium have been incriminated in increased early embryo loss.7 Luteal insufficiency is believed to be linked to thyroid dysfunction as well as obesity. Management errors such as breeding animals too early during the postpartum period (before 2 weeks) have been associated with high early embryonic death.8 Reduced ovulation rates and embryonic viability may be seen in females in a negative energy balance because of heavy milk production during the first 2 to 4 weeks of lactation. Attaining a diagnosis of the cause of early pregnancy loss is most likely one of the most challenging and frustrating exercises in practice.
Specific Disorders of the Reproductive System in the Female
Ovarian Disorders
Ovarian disorders are the second most common cause of reproductive failure and represent about one third of all genital diseases in camelids in our theriogenology referral service (Table 20-3).9 Many females may present with uterine infection as the main complaint, whereas the primary cause of infertility may be failure of conception because of ovarian or uterotubal problems. Precise diagnosis of ovarian and tubal diseases requires a methodical approach, a good understanding of the reproductive anatomy and physiology, and mastery of transrectal imaging of the reproductive organs with ultrasonography. It is important to communicate to the breeder that diagnosis of the cause of infertility, in general, and that of ovaries, in particular, may require several examinations.
TABLE 20-3
Reproductive Disorders by Organ Diagnosed over a 13-Year-Period in Female Alpacas and Llamas*
*Presented for infertility at the Theriogenology service of the Veterinary Teaching Hospital at Washington State University.
Adapted from Tibary A, et al: An approach to the diagnosis of infertility in camelids: retrospective study in alpaca, llamas and camels, J Camel Pract Res 8:167-179, 2001; Tibary A, et al: Reproductive disorders in alpacas and lamas at a referral center, Reprod Domestic Anim 45:110-110, 2010.
Congenital Abnormalities of the Ovary
Congenital hypoplasia of the ovaries is relatively common in infertile maiden females. Ovarian hypoplasia or dygenesis was found in 16.8% of 155 infertile alpaca females on postmortem examination in an abattoir in Peru.10 A case series study showed that the majority of these females present with a complaint of continuously receptivity.11 Physical examination is often unremarkable except for a predisposition of the animals to being taller and “leggier” than normal. Diagnosis is based on absence of follicular activity verified by serial ultrasonographic examinations (3 examinations at 3-day intervals). Ultrasonography of the reproductive organs shows a small infantile and flaccid uterus. The ovaries are often so small that they cannot be visualized even by the most experienced examiner. Vaginal examination may reveal a flaccid, open cervix. Serum estrogen levels may be used to monitor follicular activity. Samples should be taken every 3 days for 10 to 12 days. Follicular development may be seen in some females, but the follicles fail to develop to an ovulatory size.1 In true ovarian hypoplasia or gonadal dysgenesis, the ovary is extremely small (2 × 3 mm instead of 8 to 15 mm) and the ovarian tissue is devoid of any germinal epithelium (Figure 20-1). Confirmation is best done by laparoscopy (see Figure 20-1). The etiopathogenesis of gonadal dysgenesis is not known, but some concerns exist about its association with some lines of alpacas, particularly with males with small testicles. The karyotype may be abnormal in some cases (X0, XX/XY, XXX). Some females have been identified with a translocation resulting in a minute chromosome.11
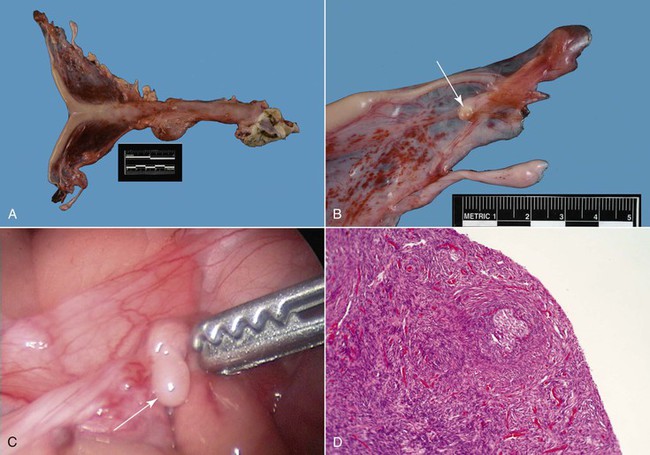
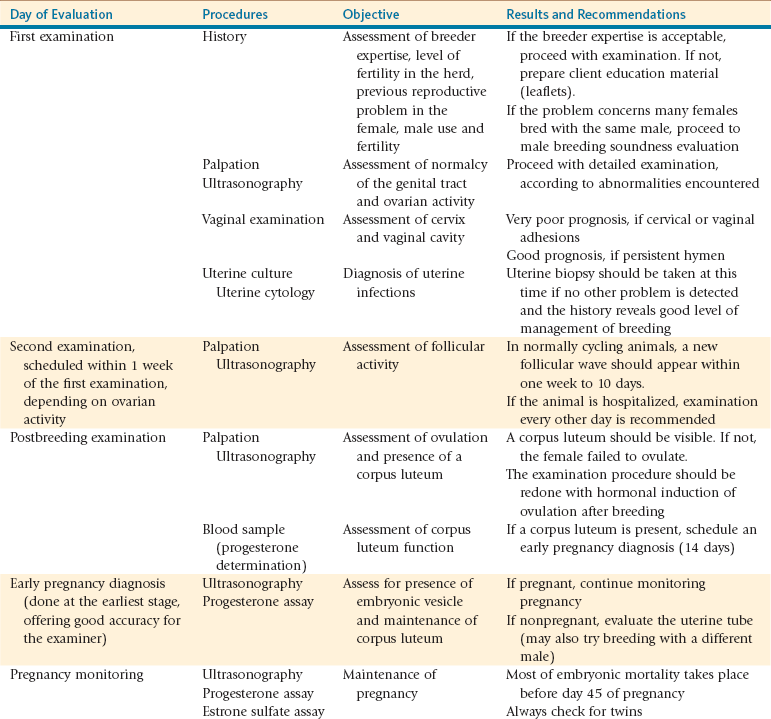
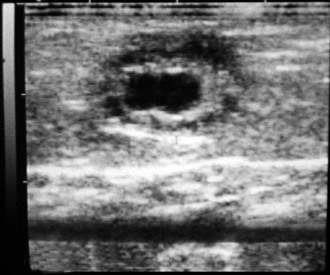
Figure 20-1 Ovarian Hypoplasia in a 22-Month-Old Alpaca Female with a History of Repeat Breeding.
A, Complete reproductive tract; note the small flaccid uterus. B, Close up view of the ovary (arrow) measuring 3 ×2 mm. C, Laparoscopic diagnosis; note the smooth surface of the surface of the ovary (arrow). D, Ovarian tissue histology; note the absence of any follicular development. (Reprinted with permission, Tibary A and A Anouassi A, Theriogenology in Camelidae, second edition, Actes Editions, Institut Agronomique et Veterinarie Hassan II, Rabat, Morocco.)
Other congenital abnormalities of the ovary include cystic rete ovarii and congenital ovarian teratomas. Cystic rete ovarii has been diagnosed in a few alpacas with absence of normal follicular development. The exact nature of this pathology remains to be studied (Figure 20-2).
Acquired Ovarian Disorders
Acquired conditions include ovarian inactivity (anestrus), persistent luteal activity, ovulation failure, luteal insufficiency, cystic conditions, ovarian neoplasms, and ovariobursal inflammations. In an abattoir study of 155 infertile alpacas, Sumar found follicular cysts, ovarian tumors, and ovarian abscesses in 8.4%, 3.2%, and 1.2%, respectively, of the cases examined.10
Cystic Conditions of the Ovary
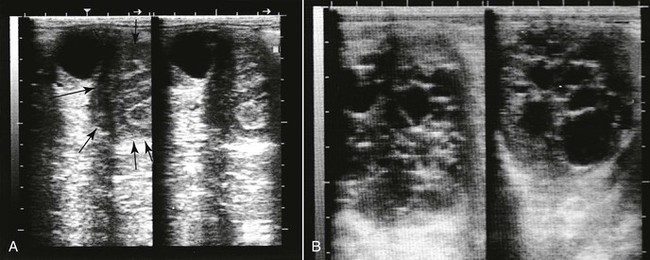
Although cystic conditions of the ovary have been described in camelids, the term cystic ovarian disease, as applied in ruminants, may not be appropriate for use in camelids because a large proportion of nonmated female camelids may develop anovulatory follicles. Ovarian cysts are traditionally classified as follicular cysts, luteal cysts, cystic corpora lutea, and hemorrhagic cysts, according to their ultrasonographic, histologic, and endocrinologic characteristics (Figure 20-3). Cystic corpora lutea are normal-functioning cavitary corpora lutea. Anovulatory or cystic follicles are defined as persistent follicular structures of more than 12 mm or more than 14 mm in alpacas and llamas, respectively. Anovulatory follicles may be single or multiple and may reach sizes of up to 70 mm. They are usually spherical, projecting from the ovary, and easily identifiable by ultrasonography (see Figure 20-3.). The incidence ranges from 8% to 30%.4,12 A progression seems to exist in some nonmated females from development of large anovulatory follicles to a hemorrhagic or luteinized status. Hemorrhagic follicles present a characteristic appearance on ultrasonography and may become luteinized over time and present a thicker wall. Some females seem to be more predisposed to the development of anovulatory follicles on successive follicular waves. True cystic ovarian disease may develop following superstimuation of the ovaries by follicle stimulating hormone (FSH) or equine chorionic gonadotropin (eCG). The effect of these cysts on fertility has not been thoroughly investigated. It is important to note that ovarian follicular activity is maintained in the majority of cases. Females with luteinized hemorrhagic or anovulatory follicles may present for persistent rejection of the male because of significant production of progesterone.
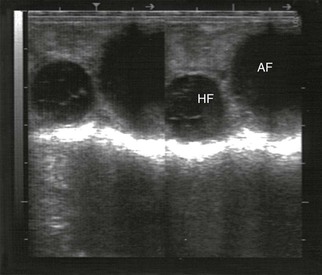
Figure 20-3 Ultrasonogram of a llama ovary with one anovulatory follicle (AF) and one hemorrhagic anovulatory follicle (HF).
Ovarian Inactivity
Acquired abnormal follicular activity may take one of three forms: (1) absence of follicular waves, (2) incomplete follicular waves (lack of development to an ovulatory size), or (3) lack of ovulatory response. These problems are primarily observed in lactating and aged females. Poor body condition or severe weight loss related to lactation, poor nutrition, or a severe debilitating disease is often associated with reduced ovarian activity. Specific trace mineral insufficiencies (selenium, phosphorus) are suspected causes of ovarian inactivity. Abnormal ovarian follicular development is also seen in obese females. Excessive use of exogenous hormones such as GnRH, progesterone, and estrogen may also inhibit normal follicular development. Clinical diagnosis of ovarian inactivity is based on serial ultrasonography, estrogen profiles, and laparoscopy.1,3,13 Stimulation of follicular development with FSH or eCG may be used to determine the degree of ovarian inactivity. If the ovaries are normal, follicular development and growth to the ovulatory size (8 mm) is seen usually about 6 to 8 days following treatment.
Ovulation Failure
Ovulation failure may be caused by inadequate luteinizing hormone (LH) release after copulation. Diagnosis of ovulation failure requires serial ultrasonography, mating with a proven male, and verification of ovulation by ultrasonography or progesterone determination 7 to 8 days following mating or administration of an ovulation-inducing drug (hCG, 500 to 1000 IU, IV; or GnRH 25 to 50 mcg, IV or IM). Although described in camelids, ovulation failure caused by female factors when the ovary appears normal may not be as common as previously thought. Ovulation failure is primarily caused by bad timing of breeding in relationship to follicular development (breeding during regression or before the follicle has acquired its ability to respond to LH). We have suspected that some males with low fertility may have a lower concentration or potency of the ovulation-inducing factor (OIF). Short copulation times may also be implicated in lack of ovulation. It is interesting to note that short copulation times (<10 minutes) followed by administration of GnRH result in high ovulation rates and good conception rates. Chronic uterine disease may be implicated in failure of ovulation because of lack of absorption of OIF. Overuse of estrogens may cause ovulation inhibition. A single injection of estradiol cypionate (ECP) may affect levels for up to 10 days. This is one reason why we no longer recommend the use of estrogen to help open the cervix for diagnostic procedures.
Persistent Luteal Activity
Treatment consists of administration of a luteolytic dose of PGF2α. The PGF2α analogue cloprostenol is the drug of choice and is given intramuscularly at a dose of 125 to 250 mcg. Natural PGF2α (dinoprost tromethamine) may be used at the dose of 5 mg IM but has been associated with some reactions in a few animals (notably, respiratory distress). We have used dinoprost tromethamine at the dose of 1.5 mg IM twice daily with no adverse reaction. Females will generally show strong receptivity within 2 to 5 days of the last injection. Reoccurrence of the condition is not uncommon.14
Luteal Insufficiency
Luteal insufficiency is often suspected in the presence of a history of recurrent early pregnancy loss between day 25 and day 60 of gestation. Studies on the incidence of primary luteal insufficiency are not available. Poor CL development is suspected in lactating females.15 Lactating females tend to have smaller follicle size.16 The roles of “stress,” fiber production, and genetics in poor luteal activity remain to be studied. A negative relationship exists between circulating progesterone levels and liver metabolism and obesity. Various treatments have been suggested on the basis of supplementation with various progestogens in the form of implants or injections (medroxyprogesterone, hydroxyprogesterone, megestrol acetate, etc.).17 To date, in our laboratory, we have been able to demonstrate that natural progesterone, hydroxyprogesterone caproate, and altrenogest may be used to maintain pregnancy in females with luteal insufficiency. Altrenogest is only effective when administered in the injectable form. Oral altrenogest is not effective. Norgestomet implants are available in some countries and seem to be effective. Because pregnancy maintenance relies on ovarian progesterone, treatment needs to be continued throughout pregnancy. Most preparations used last about 2 to 3 weeks. Treatment should be stopped at day 300 of pregnancy to avoid complications (i.e failure or cervical dilation, poor lactation).
Ovarian Neoplasia
Ovarian tumors have been reported in 3.2% of infertile alpacas examined postmortem.10 The most commonly reported ovarian neoplasms are teratoma and granulosa theca cell tumors (GTCTs). Teratomas are often an incidental finding and do not affect reproductive function unless they are bilateral. Ovarian teratomas appear often as solid masses on ultrasonography (Figure 20-4) because of the presence of various dense tissue (skin, hair, cartilage, bone, etc.).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree