Infectious Diseases of Domestic Rabbits
Introduction
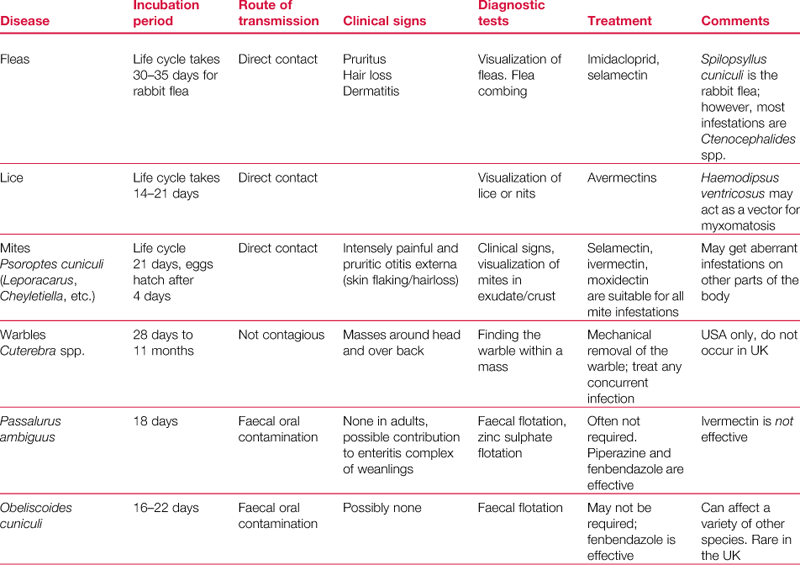
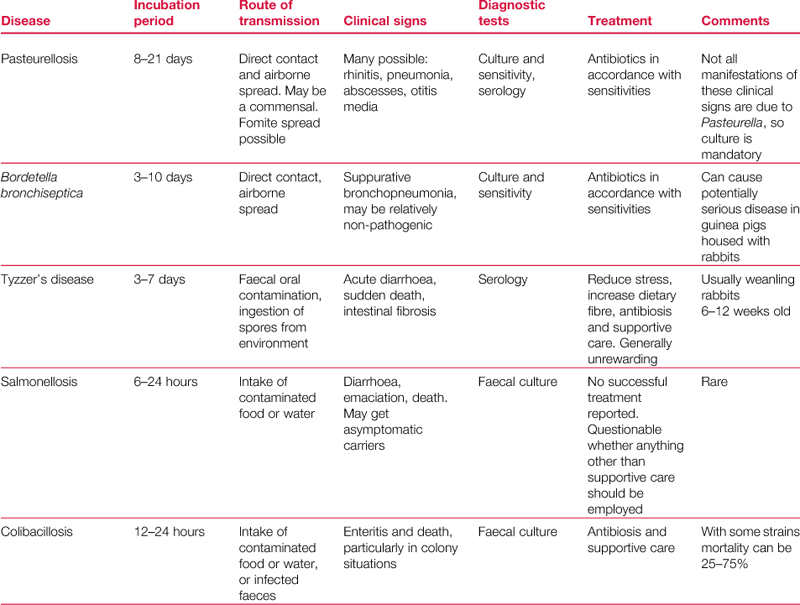
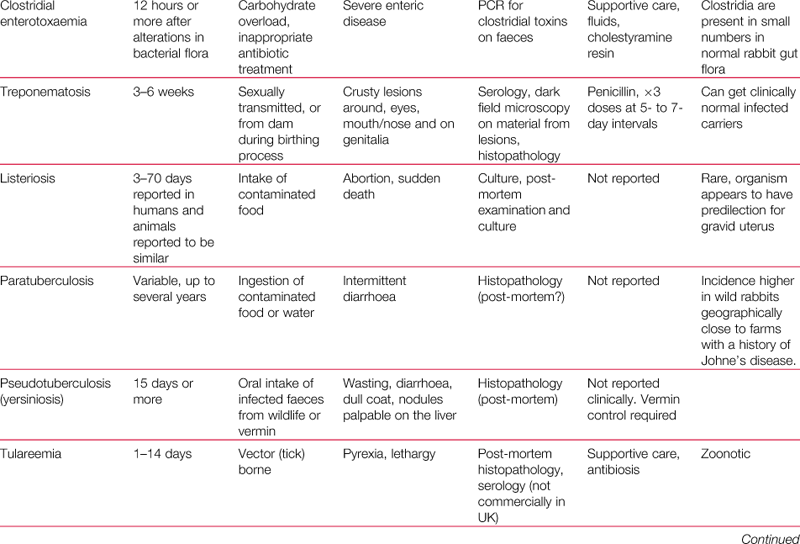
Domestic rabbits are susceptible to a number of infectious diseases: parasitic, bacterial and viral. An overview of the commoner infections may be found in Table 14.1.
14.1 Parasites of rabbits
Wild rabbits are host to a variety of parasites that can be transmitted to domestic rabbits. The type and species of parasite varies throughout the world and it is beyond the scope of this book to describe them all. A detailed, illustrated description is given by Hofing and Kraus (1994). The parasites that affect domestic rabbits are described in detail by Owen (1992). This is the major reference source for the parasite section of this chapter.
14.2 Ectoparasites
14.2.1 Fleas
Spilopsyllus cuniculi is a common flea that infests wild rabbits in Europe. It does not occur in the USA (Kraus et al., 1984). The fleas have a predilection for the ears, where they can be found in clusters along the edges of the pinnae. The fleas are mobile and move between the environment and the host. Wild rabbit fleas are not usually found on pet rabbits. Spilopsyllus cuniculi is a small flea whose life cycle is influenced by the reproductive status of the host. Egg maturation is dependent on female reproductive hormones. Successful reproduction of the rabbit flea requires contact with a rabbit in late pregnancy or with a newborn nestling. Increased blood corticosteroid concentrations in late pregnancy attract fleas, which attach firmly to the doe to feed. Within a few hours of parturition, fleas move from the doe to the newborn babies to feed, copulate and lay eggs in the nest. The eggs hatch and the larvae feed on flea dirt deposited in the nest by the adult fleas feeding on the pregnant doe. In this way, fleas are spread from one generation to the next and are an important vector of disease, especially myxomatosis.
Ctenocephalides canis or felis, the common cat and dog flea, is the usual flea found on pet rabbits. Infestation results from rabbits living in a house inhabited by dogs and cats. Infestation causes intense pruritus and allergic dermatitis can develop. Fleas and flea dirt can be found on the rabbit by combing the coat with a fine-toothed comb.
Control of flea infestation is as for other species. Fipronil should not be used on rabbits; however, imidacloprid and selamectin are both safe and effective. All in-contact animals should be treated (including other species), and environmental control should be implemented.
14.2.2 Lice
Haemodipsus ventricosus is a sucking louse that affects wild rabbits and may act as a vector for myxomatosis. It is a large louse 1.5–2.5 mm in length. It is occasionally found on pet rabbits (Owen, 1992).
14.2.3 Mites
Psoroptes cuniculi is the common ear mite of rabbits that causes crusting and ulceration of the external ear canal. The mites are large and active and are just visible to the naked eye. They are surface dwellers that cause intense irritation when they are present in large numbers (see Figure 14.1). Occasionally they are found in other areas of the body such as the perineal skin folds (see Section 7.14.3.1).
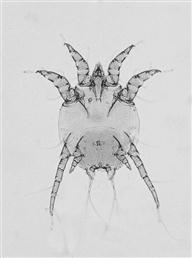
Figure 14.1 Psoroptes cuniculi.
The rabbit ear mite, Psoroptes cuniculi, causes crusting and inflammation of the external ear canal, which often extends up the pinna (see Figure 7.4). Lesions are sometimes found on other parts of the body such as the perineal skin folds. Mites can just be seen with the naked eye in exudate from the lesions. Large numbers of P. cuniculi are visible on microscopic examination of the exudate, which can be softened in liquid paraffin before placing on a glass slide. (Image supplied by Dr Sheelagh Lloyd, Division of Animal Pathology, University of Cambridge.)
Cheyletiella parasitovorax is a fur-dwelling mite that can be found in large numbers in pet rabbits (see Section 7.14.3.3). Areas of dense, flaky, encrusted skin are found along the back, especially above the tail base and on the neck. The mites are easily identified by microscopic examination of skin brushings or pluckings (see Figure 14.2). Infestation with cheyletiella is often associated with obesity, spinal disorders or dental disease. Cheyletiella parasitovorax is zoonotic and can cause erythema and pruritus in man. Pruritic lesions are found on the forearm or neck of humansthat have handled infested rabbits. The lesions regress over 24 h.
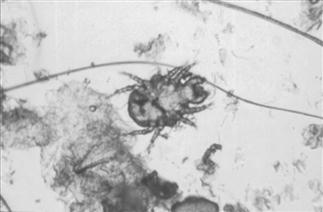
Figure 14.2 Cheyletiella parasitovorax.
This mite can be found in the fur of healthy rabbits. It is not always associated with skin lesions. In large numbers, C. parasitovorax mites cause pruritus and areas of white, flaky skin. Heavy infestation is usually linked to some underlying problem with grooming, such as dental disease, obesity or spinal disorders. Mites may be seen moving among skin flakes that are combed out and placed under a bright light. Cheyletiella parasitovorax can also be detected by combing out the flakes and applying acetate strips to the exposed underlying skin. The acetate strip is placed on a microscope slide and examined on low power. In heavy infestations a variety of nymphal stages, eggs and adult mites are seen.
Leporacarus gibbus (formerly known as Listrophorus gibbus) is the common fur mite of rabbits (see Section 7.14.3.4). Infestation is normally asymptomatic and is not significant, except that large numbers can indicate some underlying disease. The mite is usually found attached to the hair shaft where it feeds on sebaceous gland secretions (see Figure 14.3). The mites are just visible to the naked eye especially on light-coloured rabbits when infestation gives the coat the appearance of being sprinkled with pepper. This effect is more obvious when the coat is wet.


Figure 14.3 Leporacarus gibbus (formerly Listrophorus gibbus).
Leporacarus gibbus can be found in the fur of many pet rabbits. Infestation is usually asymptomatic. Like C. parasitovorax, heavy infestation is linked to some underlying problem with grooming, such as dental disease, obesity or spinal disorders. The mite is just visible to the naked eye, especially in light-coloured rabbits. A simple method of detecting L. gibbus is to comb through the fur with a fine-toothed flea comb and place the combings in a small, clear plastic bag. The contents of the bag are viewed microscopically under low power and the mites are seen moving along hair shafts. Eggs and empty egg cases can be seen attached to hair shafts. Immature and adult mites are visible. There are morphological differences between male (A) and female mites (B).
Notoedres and Sarcoptes have been described as causes of mange in rabbits.
Mites are susceptible to a range of anti-parasitic medications: selamectin (Stronghold, Pfizer) moxidectin (Advocate, Bayer) and ivermectin (many preparations). However, as many cases of mites, in particular Leporacarus and Cheyletiella, are due to inability to groom, a robust diagnostic work-up should be undertaken to look for foci of pain or inability to balance.
14.2.4 Warble flies
Cuterebra horripilum and Cuterebra buccata are warble flies that affect rabbits in the USA but do not occur in the UK.
14.3 Endoparasites
14.3.1 Intestinal worms
There is a range of nematodes that affect wild rabbits in various parts of the world. With the exception of Passalurus ambiguus, infestations in domestic rabbits are rare, especially in pets, and are unlikely to be encountered. Passalurus ambiguus is an oxyurid found in the caecum and large intestine. The adult worms measure 5–10 mm and are not pathogenic in the adult animal; indeed, they are thought to have a role in the mechanical function of the caecum. Heavy infestations in young rabbits can be a contributory factor to the enteritis complex of diseases that occur around weaning (see Section 8.2).
The small, thread-like worm is seen in the faeces of affected animals. The life cycle is direct. Passalurus ambiguus is susceptible to most anthelmintics, e.g., piperazine and fenbendazole. Ivermectin is ineffective (Morrisey, 1996). It is unlikely that P. ambiguus would require treatment in the adult rabbit. Control strategies in the environment should include restricting access to potentially infected faeces, i.e. regular cleaning of hutches, and pasture rotation, particularly where young rabbits are kept.
There are other helminth parasites that principally affect wild rabbits and are not found in the domestic pet. They include Graphidium strigosum and Trichostrongylus retortaeformis (Allan et al., 1999). Obeliscoides cuniculi occurs in wild rabbits in various parts of the world and in domestic rabbits in the USA (Hofing and Kraus, 1994). Clinically it causes haemorrhagic diarrhoea. Obeliscoides cuniculi has been used as a laboratory model of Trichostrongylus and Ostertagia species of ruminants. No species of trematode has been reported in rabbits (Kraus et al., 1984).
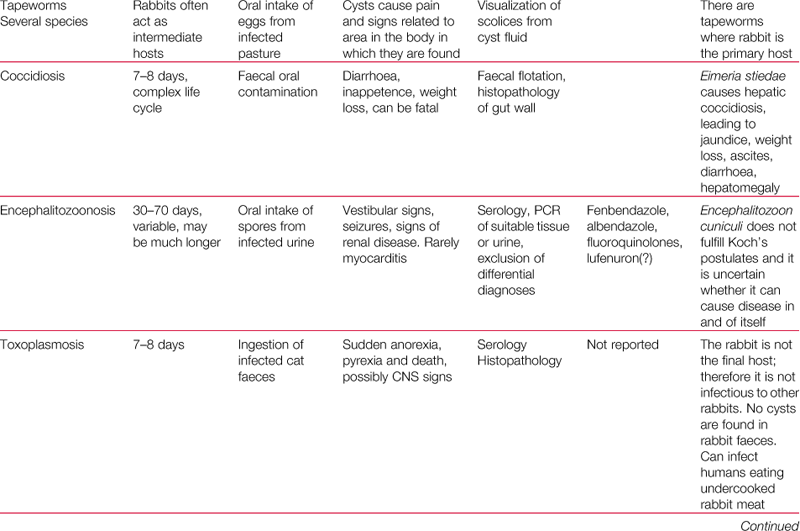
14.3.2 Tapeworms
The rabbit is the intermediate host for several tapeworms that affect dogs and cats. Pet rabbits that graze in gardens inhabited by pet dogs or visited by foxes can become infected. The incidence of these parasites is not high, as most pet owners now worm their dogs with preparations that are effective against tapeworms.
Cysticercus pisiformis is the larval stage of Taenia pisiformis, which is a tapeworm that affects dogs and foxes, with rabbits acting as the intermediate host. Tapeworm segments packed with eggs are shed in faeces and contaminate pasture. Grazing rabbits ingest eggs that pass into the small intestine where the oncosphere emerges and migrates to the peritoneal cavity via the liver. Multiple oval cysts are found in the mesentery (see Figure 14.4). The cysts contain the inverted scolex of the tapeworm. Heavy infections cause abdominal discomfort and distension. In severe cases, they can cause intestinal obstruction. Migration through the liver results in the development of fibrous tracks and necrotic foci.
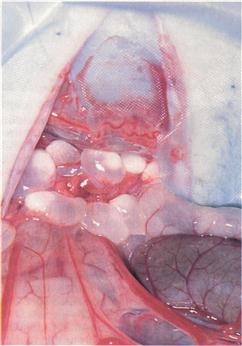
Figure 14.4 Cysticercus pisiformis.
Cysticercus pisiformis is the larval stage of Taenia pisiformis, which is a tapeworm that affects dogs and foxes, with rabbits acting as the intermediate host. Multiple oval cysts are found in the mesentery. The cysts contain the inverted scolex of the tapeworm. Some of the cysts found during an exploratory laparotomy of an anorexic rabbit showing signs of abdominal discomfort is shown. No faeces had been passed for 48 h. The rabbit was a mature angora male that had recently been adopted by a rescue centre. The cysts were most abundant in the mesentery between the stomach and the distal colon. The cysts had become so large that they had obstructed the large intestine.
Coenurus serialis is the larval stage of Taenia serialis, which is a tapeworm that affects dogs and foxes. A variety of mammals can act as intermediate hosts, usually wild rabbits and hares, but primates and even man can host the intermediate stage. Oncospheres from this tapeworm migrate to the subcutaneous tissue where they form cysts that are palpated as soft swellings under the skin. The cyst contains fluid and inverted secondary buds, each containing a scolex. Occasionally a cyst may be found in the orbit where it causes a retrobulbar swelling (Wills, 2001).
Echinococcus granulosus affects dogs and foxes. Most mammalian species, including man and rabbits, can act as intermediate hosts. The adult tapeworm is small in comparison to other tapeworms. It measures 2–9 mm. Oncospheres from ingested eggs migrate to the liver or the lung via the mesenteric blood vessels. The oncosphere then develops into a huge cyst that is able to produce secondary buds, each with an inverted scolex that can produce daughter cysts. The daughter cysts can, in turn, produce daughter cysts, with the result that a huge cyst full of smaller cysts develops. Rupture of the cyst seeds the surrounding tissues with smaller cysts, all of which are capable of developing.
The rabbit can also be a primary host for tapeworms. The cestode species varies in wild rabbits from different parts of the world. An example is Cittotaenia ctenoides, which has a free-living mite as its intermediate host.
14.4 Protozoa
14.4.1 Coccidiosis
There are at least 14 species of Eimeria which affect rabbits and vary in pathogenicity. Coccidiosis can be a serious problem in rabbit colonies. The disease is described in Section 8.10.1. Eimeria magna and Eimeria irresidua are the two most pathogenic species that affect the intestine. Other less pathogenic species include Eimeria perforans, Eimeria media, Eimeria elongata, Eimeria neoloporis, Eimeria intestinalis, Eimeria caecicola and Eimeria piriformis.
Eimeria stiedae causes hepatic coccidiosis and has a slightly different life cycle from the intestinal Eimeria species. Oocysts can survive for many years in the environment but are susceptible to dry conditions. Recovered rabbits become immune to infection.
14.4.2 Encephalitozoon cuniculi
Encephalitozoon cuniculi is a spore-forming obligate intracellular parasite belonging to the phylum Microspora (Wasson and Peper, 2000). The criterion for inclusion in this phylum is possession of a ‘polar filament’, which extrudes as the spore germinates and is thought to help gain entry into host cells. There are several Encephalitozoon species (e.g., Encephalitozoon intestinalis, Encephalitozoon hellem, Encephalitozoon bieneusi, Encephalitozoon septata) most of which are opportunist pathogens in immunocompromised human hosts. Diarrhoea, renal disease and keratoconjunctivitis are among the diseases that have been associated with encephalitozoonosis in humans. In animals, E. cuniculi is the most important member of the order Microsporidia. Encephalitozoon cuniculi primarily affects rabbits but can be found in other species. Microsporidia are unusual in that they lack mitochondria, presumably gaining their nutrition from the host cells (Pakes and Gerrity, 1994). They are characterized by a firm capsule that is strongly Gram-negative (Owen, 1992). A long polar filament is neatly coiled within it. The spore has a polar cap. Infection of the host usually occurs by oral ingestion of food contaminated with infected urine. Once in the alimentary tract, the spore comes in close contact with the mucosa and infects a host cell by extruding the polar filament. Sporoplasm is transferred through the polar filament into a vacuole in the host cell where multiplication takes place. Dividing organisms are lined up along the vacuolar membrane that is thought to be of host origin (Pakes and Gerrity, 1994).
Although E. cuniculi is considered to be protozoal, the presence of chitin and trehalose, which are also components of fungi, suggests the relationship to the fungi may be closer than previously thought (Wasson and Peper, 2000). It is a ubiquitous organism, with a wide host distribution, having been isolated from rabbits, shrews mice, rats, hamsters, muskrats, guinea pigs, goats, sheep, pigs, horses, domestic dogs, domestic cats, both wild and captive foxes, non-human primates and man (Didier et al., 2000; Wasson and Peper, 2000). Three strains of E. cuniculi are recognized. Strain I affects rabbits, strain II affects rodents and strain III affects dogs. These strains may be distinguished on a molecular level (Didier et al., 1995).
Infection is by intake of spores. These may be shed in the faeces, mucus and most commonly urine of infected animals. Spores can remain viable in the environment at 22°C for at least 4 weeks (Waller, 1979). Infection of a new host may be by ingestion, inhalation or transplacentally (Baneux and Pognan, 2003). Spores then enter host cells within the gastrointestinal or respiratory system, targeting reticuloendothelial cells in particular. It is unclear whether this is solely by extrusion of the polar tube or if phagocytosis also plays a role. The cells of the reticuloendothelial system are among those invaded and they distribute the parasite around the body. Eventually the organisms develop into mature spores that are oval in shape and measure approximately 2.5 × 1.5 μm with a thick cell wall (Pakes and Gerrity, 1994). The vacuole becomes distended and the cell eventually ruptures, releasing spores that invade new cells. Rupture of the cells is associated with an inflammatory response (Pattison et al., 1971).
Due to the early appearance of the organism in the blood, intradermal testing for E. cuniculi becomes positive at 7 days post infection and antibody activity is measurable at 14–28 days post infection (Kunstyr and Naumann, 1985; Pakes et al., 1972).
Once within the bloodstream, the organism is disseminated to areas of high blood flow initially and by 31 days after infection it can be detected in the kidneys, liver and lungs (Percy and Barthold, 2001). Once suitable host cells are penetrated, the parasite proliferates (merogony) and differentiates and matures (sporogony), causing eventual rupture of the host cell and release of spores to complete the life cycle. It is postulated that the rupture of host cells and release of foreign material initiates the granulomatous response commonly associated with this disease. This is why histopathological examination frequently fails to find evidence of organisms within the granulomatous lesions. Chronic granulomatous inflammation in these organs is thought to be responsible for the clinical signs attributed to E. cuniculi. The clinical signs commonly believed to be a result of encephalitozoonosis may be grouped into three broad categories: signs of central nervous disease, signs of renal disease and those of ocular disease. Chronic inflammation results in the development of granulomatous lesions in target organs, primarily the kidney and brain, although the liver may be involved (see Figure 10.6 and Figure 14.5). Myocarditis has also been reported (Pakes and Gerrity, 1994). Clinical signs are associated with granulomatous encephalitis or nephritis, notably vestibular disease and chronic renal failure. Encephalitozoon cuniculi can also cause lens rupture, pyogranulomatous uveitis and cataracts in rabbits (see Section 9.7.3.1 and Figure 9.4). In utero infection of the lens in the developing embryo occurs and causes the lens to rupture in later life (Stiles et al., 1997).
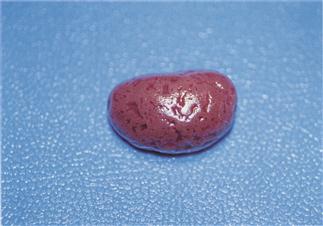
Figure 14.5 Kidney showing gross lesions associated with Encephalitozoon cuniculi infection.
The kidney of a 4-year-old male dwarf lop rabbit known to be seropositive for Encephalitozoon cuniculi although he showed no obvious clinical symptoms is shown. Both kidneys showed irregular, depressed areas. Encephalitozoon cuniculi causes granulomatous interstitial nephritis. Long-standing lesions show interstitial fibrosis and collapse of the parenchyma. Early lesions show focal granulomatous inflammation. Lesions are present in the renal tubule and spores are shed in the urine, which is infective to other rabbits.
Urinary shedding of spores starts at day 42, by which time the organism has localized within the renal tubular cells, and shedding is at its greatest by day 56. By day 63 the antibody response is at its maximum (Harcourt-Brown and Holloway, 2003). Only after 63–70 days are the organisms shown to be present and causing lesions in the brain, though compared to the lesions seen in the kidney at this stage, these are relatively mild. From this timeline it is apparent that it is possible to have a positive titre to E. cuniculi before the organism is likely to have entered the central nervous system. Any rabbit showing central nervous signs at this time could be wrongly supposed to be showing clinical signs due to this disease. The ability to differentiate IgM and IgG titres may clarify this situation. IgM titres increase early in the course of an infection, then fall, and are absent after day 38. IgG rises more slowly but remains measurable for years (Kunstyr et al., 1986, Sobottka et al., 2001).
Urinary spore shedding ceases by 90 days post infection and by day 98 the organisms are located in the organs of predilection, namely the brain, kidney and heart. Although the heart is named as an area of predilection, heart disease due to E. cuniculi is rarely diagnosed.
14.4.2.1 Encephalitozoon cuniculi in other species
Encephalitozoon cuniculi can infect a number of mammalian species with predilection sites and disease variation between hosts. Infections have been reported in rabbits, mice, guinea pigs, hamsters, dogs, cats, monkeys and man. There are no morphological or immunological differences between strains of E. cuniculi affecting laboratory animals.
Encephalitozoonosis has also been reported in birds (Poonacha and Stamper, 1985). Guinea pigs housed with infected rabbits were found to be at more risk than those housed separately in a survey by Gannon (1980). Nephritis was common but cerebral granulomas were not seen in the guinea pigs. Encephalitozoon cuniculi has been described in a wild rabbit in 1955 (Pakes and Gerrity, 1994) but more recent serological surveys have failed to find evidence of infection in wild rabbits, although they can be infected experimentally (Cox and Ross, 1980; Cox et al., 1980). It has been suggested that the natural hygiene habits of wild rabbits significantly decrease post-natal infection.
Serological surveys in dogs in the UK (Hollister et al., 1989) and South Africa (Stewart et al., 1988) demonstrated prevalences of 13 and 2–23%, respectively. The latter prevalences demonstrated the difference between healthy control dogs (2% prevalence), and those with chronic renal disease (23% prevalence). The canine strain (strain III) of E. cuniculi has been shown to cause disease in humans (Weitzel et al., 2001).
Two recent studies have looked at the incidence of E. cuniculi in wild rodent populations. Hersteinsson et al. (1993) found serological evidence of the organism in wild mice in Iceland, and Muller-Doblies et al. (2002) isolated E. cuniculi from a free-ranging rat (Rattus norvegicus). Strain II is typically found in rodents. It has been suggested that carnivores are infected by eating infected prey and that the disease in wild rodent population acts as a reservoir of infection for these species.
In addition to rabbits, E. cuniculi can cause severe disease in blue (Arctic) foxes, Alopex lagopus, and financial losses to the fur industry. This disease has therefore been extensively studied in this species. Adult blue foxes are asymptomatic carriers but can pass on infection transplacentally and the resultant puppies can have various clinical signs, depending on what body system is primarily involved. These range from acute renal failure to cardiac signs to widespread non-suppurative meningoencephalitis. Akerstedt (2003) demonstrated the humoral response of adult blue foxes in Norway persists for at least one year. Disease in blue foxes has prompted serological surveys of farmed Arctic foxes in Finland (Akerstedt et al., 2002), wild Arctic foxes in Greenland (Akerstedt and Kapel, 2003) and other species of fox, Dusicyon culpaeus and Dusicyon griseus, in Argentina (Martino et al., 2004). These studies have shown that wild foxes in both Greenland and Argentina demonstrated no evidence of encephalitozoonosis within their populations. However, in farmed foxes the disease is endemic. This mirrors the situation in rabbits in the United Kingdom (Cox and Ross, 1980; Blevins, unpublished data; R. Saunders, personal communication), where exposure is common in rabbit colonies and within the pet population but not in the wild rabbits, but contrasts with the situation in both the United States (Jungherr, 1955) and Australia (Thomas et al., 1997), where exposure has been documented in wild rabbits. In the case of Australian rabbits the seroprevalence in the wild is suggested to have changed in recent years. A study in 1980 found no evidence of exposure to E. cuniculi in wild rabbits in Victoria (Cox et al., 1980), whereas a study in 1997 showed a seroprevalence of 25% in wild rabbits in Western Australia (Thomas et al., 1997). The mechanism for the shift in prevalence in the wild populations in both America and Australia is unknown. In the case of Australia it is possible that E. cuniculi was always present in wild rabbits in Western Australia, and is still not present in those in Victoria.
14.4.2.2 Zoonotic potential of Encephalitozoon cuniculi
Although E. cuniculi can infect a range of hosts, severe systemic disease is rare in other species except in athymic or immunosuppressed mice and neonatal dogs or foxes. Athymic mice do not develop a cellular or humoral response to the parasite and masses of spores are found in the liver and other viscera (Gannon, 1980). Experimental infection of rabbits with E. cuniculi cultures administered into the rectum with a catheter after weeks of repeated colonic enemas resulted in E. cuniculi infection with hepatic lesions predominating rather than the typical brain and kidney changes (Fuentealba et al., 1992).
In recent years the topic of E. cuniculi has received renewed interest due to its potential to cause disease in immunosuppressed humans. Weber et al. (1997) described a case of cerebral microsporidiosis in an individual with human immunodeficiency virus (HIV) infection, and two similar cases in children have been described (Didier, 2000). Refractory diarrhoea, bronchitis, pneumonia and sinusitis are other possible manifestations. Hollister et al. (1991) found that there was serological evidence of widespread human exposure to E. cuniculi. It is interesting to note that in the USA the prevalent strain of E. cuniculi found in humans was strain III, which is usually associated with dogs, whereas in Europe strain I (the rabbit strain) was more commonly found (Didier et al., 1995). With the discovery of HIV in the 1980s and the ever-increasing number of people undergoing chemotherapy and organ transplantation the relevance of E. cuniculi within the pet population as a potential zoonosis cannot be overstated.
14.4.2.3 Clinical signs associated with Encephalitozoon cuniculi infection in rabbits
Encephalitozoon cuniculi was first described by Wright and Craighead in 1922 in rabbits exhibiting hind leg paralysis and other neurological signs. Kimman and Akkermans (1987) described an outbreak in a colony of laboratory rabbits that resulted in heavy losses. Affected animals showed muscular weakness, emaciation, polydipsia, polyuria and occasional neurological signs. Other texts describe encephalitozoonosis as a chronic, latent disease of rabbits that is significant because of its effects on experimental results. Infection with E. cuniculi in laboratory rabbits has caused many problems to scientific studies. Subclinical infection has vitiated experimental results and lesions caused by the parasite have been wrongly attributed to a number of other ailments (Wilson, 1979). Encephalitozoonosis can interfere with test results. Blood samples of rabbits with spontaneous encephalitozoonosis have been shown to have significantly lower levels of catecholamines than healthy rabbits (Levkut et al., 1997). Nowadays, laboratory rabbits are screened for E. cuniculi and seropositive animals eliminated. In the laboratory setting a ‘test and remove’ policy in conjunction with strict hygiene is frequently employed because infection with E. cuniculi can result in erroneous results during research trials (Ansbacher et al., 1988). This is due to the fact that E. cuniculi modifies the way the central nervous system reacts to challenge.
Encephalitozoonosis is widespread in pet rabbits. There is a range of manifestation signs from acute neurological disaster to latent infections that do not exhibit clinical signs of disease. In Germany, a serological survey of 277 pet rabbits showed that 41% were seropositive (Ewringmann and Göbel, 1999). Of the seropositive rabbits, 51 (40.8%) showed clinical signs of encephalitozoonosis. In the UK, a random survey of 30 pet rabbits revealed 8 seropositive individuals (Carmichael, Idexx and Harcourt-Brown, unpublished data). After the animals had been found to be seropositive, the owners were questioned and four reported vague symptoms such as head nodding or swaying at rest, deafness or impaired mental ability. A survey of 97 clinically healthy UK rabbits in 2006 showed that 52% were seropositive (Keeble and Shaw, 2006). Samples were taken as part of a routine health screen or preanaesthetic screening at veterinary practices in England, Wales and Scotland. Veterinary surgeons completing the survey were asked for information on the animals’ husbandry, diet, vaccination, preventive medicine routines and health status. None of these factors were found to be associated with the serological status of the rabbits.
When clinical signs occur, they are usually associated with granulomatous lesions in the brain, kidney or lens, although the liver, heart and other organs can be affected. In the German survey of 277 rabbits, 51 (40% of the seropositive animals) showed signs relating to infection. Twenty-three rabbits suffered from CNS disorders, 16 from renal disease and 7 from uveitis. Two rabbits had both CNS and renal disease and 3 animals had CNS symptoms, renal disease and uveitis (Ewringmann and Göbel, 1999). Renal disease associated with E. cuniculi is described in Section 14.5.1 and ocular disease in Section 9.7.3.1.
14.4.2.4 Diagnosis of Encephalitozoon cuniculi
Since the first report of E. cuniculi as a cause of ‘infectious motor paralysis’ in young rabbits (Wright and Craighead, 1922) there has always been uncertainty surrounding the diagnosis of E. cuniculi as a cause of clinical disease in rabbits. Recent work has shown the seroprevalence of E. cuniculi in UK pet rabbits to be 52% (Keeble and Shaw, 2006). The infection is common; however, the rate of disease is unknown. Historically E. cuniculi has been found frequently in mammals, but its relevance has been misinterpreted. The use of infected rabbits as models for human disease has led to the clinical and histological signs of E. cuniculi being mistaken for syphilis and poliomyelitis (Bull, 1917). It has also been implicated at various times as the causative agent of rabies, scrub typhus, psittacosis and chemical carcinogenesis (Wasson and Peper, 2000).
Because of the significant effects this organism can have on research trials and the need to distinguish infected rabbits from those which are not, reliable serological tests are now available. These are equally suitable for use on pet rabbits as well as in the laboratory setting. The majority of research into this disease has been carried out on laboratory rabbits. With an increase in the popularity of rabbits as pets and the number presented to veterinary surgeons for treatment, it is important to consider this disease from the pet rabbit perspective. In the domestic setting it is often not enough to be able to say a rabbit is infected; it is necessary to decide whether this is relevant to the health status of the individual concerned. A positive titre only reflects exposure but gives no indication of whether the disease is active. This is important in the treatment of the individual animal, but also has relevance to the human owners, since E. cuniculi is a potential zoonosis (Deplazes et al., 1996; Weber et al., 1997; Weitzel et al., 2001).
Currently the gold standard for diagnosis of E. cuniculi is post-mortem histological examination with immunochemical staining for accurate identification of any organisms found (Franzen et al., 1999; Percy and Barthold, 2001). Changes typically associated with infection are granulomatous lesions, the distribution being defined by the time between exposure and histological examination. Central nervous system lesions are described as ‘focal non-suppurative granulomatous meningoencephalomyelitis with astrogliosis and perivascular lymphocytic infiltration’ (Percy and Barthold, 2001), and those in the kidneys are ‘focal to segmental granulomatous interstitial nephritis’ (Percy and Barthold, 2001). In both cases, traces of the organism are not reliably present in the lesions, although they may be found in adjacent cells.
In vivo testing must be compared against histological examination to evaluate the sensitivity and specificity of the results. However, in many cases, certainly those with neurological signs, direct comparison of testing with the gold standard is impossible, since the individuals being tested are still alive. In the pet rabbit, a humoral response to E. cuniculi infection cannot be relied upon for accurate diagnosis. In laboratory rabbits, serum antibodies develop after 3 weeks and excretion of the parasite occurs 6 weeks after experimental infection with E. cuniculi (Cox et al., 1979). Passive immunity is transferred from infected dams to their offspring, which can have titres of 1:25 to 1:800 that last until they are about 4 weeks old. After a seronegative period, young rabbits seroconvert at 8–10 weeks of age in response to natural infection (Lyngset, 1980). Therefore the presence of antibodies only indicates exposure to the organism and does not confirm E. cuniculi as a cause of disease.
We know that many domesticated rabbits have positive titres; however, this only indicates exposure and indeed absolute titres have no significant relationship to presence of organism in the brain, severity of clinical disease or outcome (Keeble and Shaw, 2006; Kunstyr et al., 1986). Titres may be so variable even between rabbits matched for age, breed and environment (Kunstyr et al., 1986) as to render them uninterpretable. Experimentally, high antibody titres have been found in rabbits showing signs of chronic infection (Pye and Cox, 1977). IgG titres reached a level of 160–2560 after a latent phase of 13–28 days in a study of rabbits experimentally infected with E. cuniculi (Kunstyr et al., 1986). Some of the rabbits showed an episodic humoral response and became seronegative after a few weeks. There was wide individual variability in antibody response but the authors suggested that differences in IgM and IgG could distinguish between recent and chronic infection. IgM seroconversion occurs at the beginning of the antibody response and simultaneous IgG and IgM detection suggest recent infection. Jeklova et al. (2010) have suggested that finding an IgM titre indicates active infection and warrants treatment.
The situation is made even more complicated by the fact that concurrent disease can affect the host’s immune response. Cox (1977) showed that rabbits already infected with E. cuniculi exhibited altered immune responses to intercurrent infections, and that IgG levels relating to the new infection may be depressed and IgM levels elevated compared to uninfected animals. This increases the likelihood that concurrent clinical disease may occur (Harcourt-Brown and Holloway, 2003). Similarly, infection with E. cuniculi can affect the way the central nervous system reacts to outside challenge. Ansbacher et al. (1988) showed that seropositive rabbits displayed an inconsistent inflammatory response both between individual rabbits and between sites within the same rabbit when coated platinum wires were implanted into four sites within the cerebral cortex. The response of each rabbit becomes unpredictable, and renders them unsuitable for many research projects. It is conceivable that response to intercurrent disease may be equally unpredictable.
Various serological tests are available for screening rabbits for E. cuniculi, largely due to the fact that the laboratory industry requires negative rabbits for many of its research studies. Boot et al. (2000) compared several commercially available test methods for determining their sensitivity and specificity relative to each other. Two indirect immunofluorescence assays (IIF), two enzyme-linked immunosorbent assays (ELISA) and carbon immunoassay were compared. The results suggested that there was no difference between the assays in respect to detecting positive cases, but that quantitative determinations should be performed by IIF and not ELISA. This is proposed to be due to the less quantitative nature of the ELISA assay and not due to any reduction in sensitivity relative to the other methodologies. Intradermal testing (Pakes et al., 1972) is not routinely used for screening at this time, although it proved to be both sensitive and specific in determining rabbits that were positive to the organism.
Encephalitozoon cuniculi organisms can be found in the urine of infected animals (Pye and Cox, 1977). The spores are evident as ovoid, Gram-positive organisms approximately 1.5–2.5 μm in size. Staining procedures using carbol fuchsin will stain the organisms a distinct purple colour (Percy and Barthold, 1993). Theoretically, urine examination is a means of confirming the presence of antigen in the live animal, although it is impractical as a routine diagnostic technique in general practice. Organisms are intermittently excreted and urine collection can be difficult. Normal rabbit urine often contains sediment. PCR testing is now available commercially to identify E. cuniculi in urine, faecal and tissue samples.
In the pet rabbit a clinical diagnosis of encephalitozoonosis is reached by a combination of a positive titre with suggestive clinical signs and elimination of alternate causes of these clinical signs where this is possible. The clinical work-up should include a physical examination, complete blood count and differential, biochemistries, radiography of the axial skeleton and skull, urinalysis and serology as a minimum. This should enable the clinician to rule in or out many of the possible differential diagnoses and will further direct the management of the individual case.
The major differentials for central nervous disease in the pet rabbit are pasteurellosis, neoplasia, trauma, lead poisoning and toxoplasmosis. In the USA cerebral larval migrans caused by Baylisascaris is also a differential. As encephalitozoonosis is a disease related to granuloma formation, clinical pathological evidence of this may be used to distinguish it from the possible alternate diagnoses. Of the above list only toxoplasmosis is likely to produce granulomatous lesions at the time of clinical signs becoming relevant. This disease is rarely diagnosed in UK rabbits and its significance can be eliminated by looking at paired titres for this organism. Similarly, the differential diagnoses for chronic renal failure are intrinsic renal failure, benign tumours, such as embryonal nephroma, renal cysts and malignant tumours, such as lymphoma and renal carcinoma. The defining feature of encephalitozoonosis is granulomatous change in the target organs, unlike the alternate causes of similar clinical symptoms. There are two challenges in determining the cause of many of these clinical signs. First is the reliance necessarily placed on invasive or post-mortem testing to achieve definitive diagnoses. Secondly, increases in absolute titres to E. cuniculi cannot be used diagnostically due to the difficulties in interpretation. Many workers doubt E. cuniculi as a cause of disease by itself (it does not in fact fulfil Koch’s postulates), feeling that in many cases, signs attributed to the parasite are in fact caused by one of the differential diagnoses. Successful treatment of the alternative disease can bring about a clinical cure. However, positive diagnosis of one of the differentials for the presenting clinical signs does not rule out active encephalitozoonosis.
Histological examination cannot reasonably be used for ante-mortem diagnosis in rabbits with central nervous disease. Kidney biopsy can be undertaken to establish a diagnosis in those rabbits showing renal signs; however, organisms and histological change may not be continuous throughout the kidney, so lesions can be missed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree