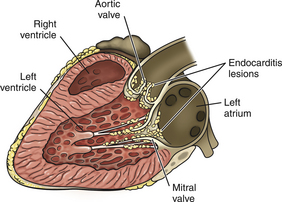
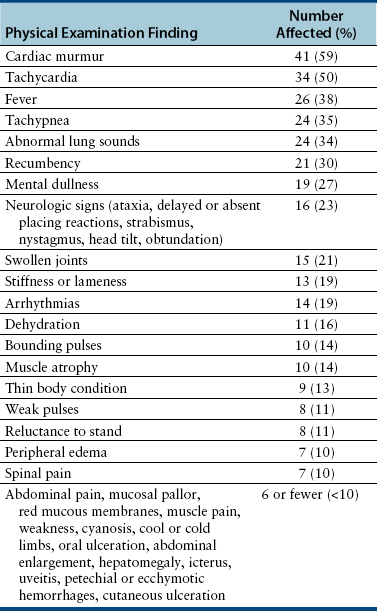
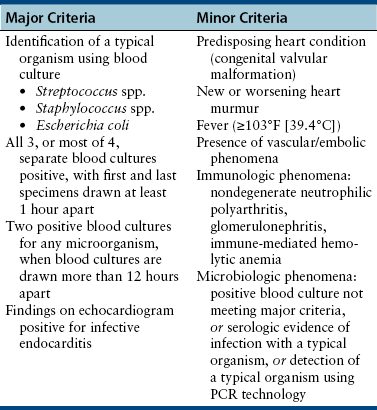
Chapter 86 Bacterial infections of the cardiovascular system include bacteremia, infective endocarditis, myocarditis, and infectious pericarditis. These infections are frequently associated with sepsis, or the host systemic inflammatory response to infection. When sepsis is associated with hypotension and distant organ dysfunction, the terms septic shock and severe sepsis are used, respectively. These terms have been clearly defined in human medicine1 and adapted for use in small animals (Table 86-1). Septicemia is a term that has been used interchangeably with bacteremia by veterinary medical and medical professionals, but it is incompletely defined, and as a result, the use of this term is no longer recommended. TABLE 86-1 Definitions That Apply to Cardiovascular Infections in Dogs and Cats Bacteremia can be divided into uncomplicated and complicated bacteremia (see Table 86-1). Transient, low-level (≤10 CFU/mL) subclinical bacteremia follows a variety of procedures such as dental prophylaxis, endoscopy, gastrointestinal surgery, and catheter placement. This is typically cleared rapidly (within 15 to 30 minutes) by a functional mononuclear phagocyte system. However, persistent release of large numbers of bacteria into the bloodstream can overwhelm the host immune response and culminate in clinically significant bacteremia. Overwhelming bacteremia can occur with disorders such as pyelonephritis, pyothorax, severe pyoderma, bacterial peritonitis, pyometra, pneumonia, pancreatitis, a variety of gastrointestinal disorders, bite wounds, orthopedic infections, or catheter-related infections. Underlying immune system dysfunction (such as occurs with diabetes mellitus, malignancy, and neutropenia) also predisposes to clinically significant bacteremia, because bacteria multiply unhindered in the bloodstream. The most common organisms isolated from the blood of sick, bacteremic dogs are Staphylococcus spp., Streptococcus spp. (especially Streptococcus canis), and Escherichia coli (Table 86-2).2 Less than 10% of bacteremic dogs in the author’s hospital are infected with anaerobes, but in a study of critically ill dogs and cats from Colorado, anaerobes were isolated from 31% of 39 dogs and 40% of 10 cats.3 With the exception of Bartonella and hemoplasmas, bacteremia is less commonly recognized in cats than in dogs and usually results from infection with gram-negative bacterial pathogens.2 Polymicrobial infections of the blood are common and typically occur in 10% to 20% of dogs and cats with positive blood culture results. Mixed infections with two or more different strains of a single bacterial species can also occur. Polymicrobial infections often occur when anaerobes are involved or when bacteremia develops secondary to compromise of the gastrointestinal tract. TABLE 86-2 Organisms That May Be Cultured Using Routine Blood Culture Methods from the Bloodstream of Dogs and Cats with Bacteremia∗ Bacteremia does not need to be present for sepsis to occur, but the clinical signs of bacteremia reflect the presence of sepsis (i.e., the host inflammatory response to infection). An understanding of the pathogenesis of sepsis has been critical for the development of drugs to improve survival, but it is complex and not fully understood. In summary, sepsis occurs when components of microbes (known as pathogen-associated molecular pattern molecules, or PAMPs) activate a host inflammatory response when they bind to pattern-recognition receptors (PRRs) such as Toll-like receptors (TLRs) and nucleotide-binding domain, leucine-rich repeat containing proteins (NLRs).3a The lipid A portion of bacterial lipopolysaccharide (LPS) is especially well known for its ability to stimulate this response (see Chapter 36), but other molecules, such as peptidoglycan, flagellin, DNA, and viral double-stranded RNA, may also be involved. Superantigens of gram-positive bacteria induce a cytokine cascade through simultaneous activation of large numbers of T cells (see Chapter 34). Bacterial LPS binds to an acute phase reactant protein called LPS binding protein, which transfers the LPS to CD14, a receptor on the surface of phagocytes.4 CD14 then transfers the LPS to TLR4, which transmits the signal to the cell interior. This leads to activation of NF-κB and the secretion of cytokines such as TNF-α and Il-1β. The released cytokines induce fever (which may serve to limit bacterial growth), and cause further lymphocyte activation and release of proinflammatory cytokines. Damaged host cells also release molecules (damage-associated molecular pattern molecules, or DAMPs) that bind to TLRs and stimulate cytokine release. Important DAMPs recognized in humans include a chromatin-associated protein known as high mobility group box-1 (HMGB1) and cellular DNA. The cascade of cytokines that is produced in response to DAMPs and PAMPs in turn induces the release and activation of a huge variety of mediators, such as complement, colony stimulating factors, prostacyclin, thromboxane, platelet-activating factor, coagulation factors, histamine, serotonin, nitric oxide, angiotensin II, and endothelin-1. Many of these mediators serve to recruit large numbers of immune cells and trigger fibrin deposition in order to localize, control, and ultimately eliminate the infection. Other molecules, such as cortisol and epinephrine, act to control the inflammatory response. Endothelial cell damage results in increased exposure of tissue factor (which activates the extrinsic coagulation pathway) as well as von Willebrand’s factor. Endothelial cell damage also results in decreased expression of thrombomodulin, an important anticoagulant that normally binds thrombin and prevents it from cleaving fibrinogen. The thrombomodulin-thrombin complex also activates protein C, which binds to protein S; the activated protein C–protein S complex degrades activated coagulation factors Va and VIIIa and prevents coagulation. Recombinant activated protein C has been the subject of much attention and controversy as a specific treatment for sepsis in human patients (see Treatment). When the inflammatory response to infection is not effectively controlled or localized, the end result is systemic hypotension, tachycardia, increased vascular permeability, leukocytosis, activation of the coagulation cascade, and disordered tissue perfusion as a result of microcirculatory disturbances, volume depletion, and depressed myocardial function. This leads to further damage to host cells, lactic acidosis, and multiple organ dysfunction. Impaired perfusion, tissue hypoxia, and endothelial cell damage may have a wide range of effects on organ function (Table 86-3). Cats may be particularly susceptible to acute lung injury in sepsis, whereas gastrointestinal and/or hepatic dysfunction is often observed in dogs. Clinical signs of severe sepsis in dogs often include fever, obtundation, tachycardia, bounding or weak pulses, bright red mucous membranes, shortened capillary refill time, icterus, and tachypnea. However, many dogs with sepsis are evaluated when in the hypodynamic phase, with tachycardia, poor pulse quality, prolonged capillary refill time, and cold extremities. Cats may show obtundation, pale mucous membranes or icterus, abdominal pain, poor pulse quality, bradycardia, and/or tachypnea and may either be febrile or hypothermic.5 TABLE 86-3 Potential Effects of Sepsis on Organ Function The reported incidence of canine IE has ranged from fewer than 0.1% of cases seen at veterinary teaching hospitals to approximately 1% of cases presenting to veterinary cardiologists.6–9 IE is uncommonly diagnosed in cats. Dogs with IE are typically middle aged to older, large-breed dogs such as German shepherds, golden and Labrador retrievers, Weimaraners, mastiffs, and Rottweilers. For unclear reasons, male dogs are affected approximately twice as often as females. Factors that predispose to IE are similar to those that predispose to continuous bacteremia. However, virulence factors possessed by bacteria subsequently affect whether IE develops, which explains why some bacterial species (especially gram-positive bacteria) are more likely to produce IE than others (gram-negative bacteria). Although valvular heart defects, especially congenital defects such as subaortic stenosis, may predispose young dogs to development of IE, the vast majority of dogs with subaortic stenosis never develop IE, and more than 80% of dogs with IE have no identifiable underlying cardiac abnormality.8,10 Furthermore, IE is rarely diagnosed in small-breed dogs, which are predisposed to myxomatous mitral valve degeneration. One study also found no association between periodontal disease and IE in dogs.10 The most commonly reported etiologic agents in canine IE are streptococci, staphylococci, and, to a lesser extent, gram-negative rods, enterococci, and Bartonella spp. (Table 86-4).7,10–13 Organisms that are less commonly incriminated include fungi, gram-positive rods, mycobacteria, and anaerobes. In dogs, the mitral and/or the aortic valves are most commonly affected (Figure 86-1). The larger, septal leaflet of the mitral valve is more commonly affected than the mural leaflet.8 The tricuspid valve and ventricular wall are rarely involved. This distribution correlates with the degree of pressure that rests on each closed valve. Classically, vegetative lesions develop along the line of valve closure. TABLE 86-4 Causes of Infective Endocarditis in Dogs FIGURE 86-1 Infective endocarditis. Vegetations are attached to the mitral and aortic valves and may be mobile or sessile. The development of IE begins with colonization of a heart valve by bacteria and deposition of fibrin, platelets, leukocytes, and erythrocytes. Colonization may be facilitated by changes in the valve surface as a result of turbulent blood flow, which results in the deposition of platelets, fibrin, and fibronectin; the end result is a sterile vegetation, or nonbacterial thrombotic endocarditis. Bacteria then adhere to this matrix, induce inflammatory cytokine and tissue factor release, and become covered with additional layers of platelets and fibrin, which promote bacterial growth to extremely high densities in an environment that is protected from the immune system. Properties of bacteria known to promote valve colonization include adhesins such as fimA in streptococci and clumping factor and fibronectin-binding proteins in staphylococci,14,15 as well as factors that cause platelet aggregation. Portions of the vegetation can then dislodge and embolize in a variety of different tissues, especially the kidneys, spleen, brain, joints, and muscles, with an enormous spectrum of associated clinical manifestations. Thromboembolic complications are more likely to occur in dogs with mitral valve involvement than in those with aortic valve IE.8 Regardless of the valve affected, valvular dysfunction can lead to impaired cardiac output and congestive heart failure (CHF). As in humans, IE in dogs may be best classified on the basis of the etiologic agent involved, because the etiologic agent often determines the clinical course of disease, disease manifestations, prognosis, and the most appropriate antimicrobial drugs for treatment. For example, Bartonella IE is more likely to involve the aortic valve than the mitral valve, often occurs in the absence of fever, has a high prevalence of CHF when compared with IE caused by other bacterial pathogens, and is negatively correlated with survival (see Figure 52-3). In contrast, Streptococcus canis IE usually involves the mitral valve and is often associated with secondary polyarthritis. Dogs with gram-negative IE may be less likely to develop CHF than those with IE caused by other pathogens.11 The clinical signs and physical examination abnormalities that occur with IE are extremely variable and depend not only on the causative organism, but also on the valve affected, the presence or absence of thromboembolic complications, and the sites and extent of thromboembolism. Common historical findings include lethargy; inappetence; locomotory problems such as lameness, shifting lameness, joint pain, stiffness, reluctance to move, and inability to walk; and vomiting, weight loss, respiratory difficulty, and/or cough. Locomotory abnormalities can result from peripheral arterial thromboembolism, embolic or immune-mediated polyarthritis, neurologic abnormalities, and rarely, hypertrophic osteopathy. The most common initial physical examination findings are fever, tachycardia, and a cardiac murmur (Table 86-5).8 In one study of dogs with IE, fever was present in the history or serial physical examinations of only 41 (60%) of 68 dogs with IE, and a cardiac murmur in only 53 (76%) of 70 dogs with IE, so an absence of fever or cardiac murmur does not rule out IE.8 Although diastolic murmurs are suggestive of IE, more than 90% of dogs with murmurs due to IE have systolic murmurs. TABLE 86-5 Physical Examination Findings in 70 Dogs with Infective Endocarditis Seen at the University of California, Davis, Veterinary Medical Teaching Hospital Modified from Sykes JE, Kittleson MD, Chomel BB, et al. Clinicopathologic findings and outcome in dogs with infective endocarditis: 71 cases (1992-2005). J Am Vet Med Assoc. 2006;228:1735-1747. Infectious myocarditis in dogs and cats usually results from hematogenous spread of microorganisms to the myocardium or extension of endocarditis lesions to the myocardium. Viruses, bacteria, protozoa, or fungi may be involved. Some organisms have a particular tropism for the myocardium (Table 86-6). Infectious myocarditis is rare in cats. TABLE 86-6 Infectious Causes of Myocarditis in Dogs and Cats Infectious pericarditis may result from direct inoculation of organisms into the pericardium (such as occurs with penetrating wounds), extension of infection from adjacent organs such as the pleural space or lungs, or hematogenous dissemination of viral, bacterial, or fungal pathogens to the pericardium. Bite wounds or grass awn migration usually result in mixed aerobic-anaerobic infections that involve organisms such as Actinomyces spp. (see Chapter 42). Infectious pericarditis as a result of hematogenous spread of pathogens is rare; idiopathic pericardial effusion and heart-base neoplasia are the most common causes of inflammatory or hemorrhagic pericardial effusion in dogs. Pathogens with particular tropism for the pericardial sac include feline infectious peritonitis virus and Coccidioides spp. (see Chapters 20 and 63). Pericarditis results in diastolic cardiac dysfunction with subsequent development of right-sided heart failure, which is manifested as weak pulses, tachycardia, jugular venous distention, ascites, and pleural effusion. Muffled heart sounds may be detected on cardiac auscultation as a result of pericardial and/or pleural effusion. Antemortem diagnosis of bacteremia is based on culture of bacteria from the bloodstream. Reasons to consider blood culture are shown in Box 86-1. The diagnosis of sepsis is based on the presence of the systemic inflammatory response syndrome (SIRS) together with suspected or proven infection (see Table 86-1). A number of biomarkers have been investigated for discrimination of sepsis from SIRS in dogs,16–18 but as yet, highly sensitive and specific biomarkers for sepsis have not been identified. The diagnosis of IE is based on a set of criteria modified from the Duke criteria used for diagnosis of IE in human patients, which consider the results of blood culture and echocardiography as major criteria, and other clinical manifestations of IE as minor criteria (Table 86-7).8,19,20 Diagnosis of infective pericarditis relies on echocardiography and the results of cytologic examination and culture of pericardial fluid, or alternatively, histopathologic examination and culture of pericardial biopsies obtained during thoracotomy or thoracoscopy. In dogs and cats, infectious myocarditis is usually a necropsy diagnosis because myocardial biopsy is rarely performed. However, bacterial myocarditis may be suspected based on echocardiographic abnormalities, the presence of arrhythmias, and positive blood cultures. TABLE 86-7 Modified Duke Criteria Used for Diagnosis of Infective Endocarditis in Dogs and Cats8,20 Definite endocarditis: 2 major criteria, or histopathologic confirmation of endocarditis. Possible endocarditis: Positive echocardiographic findings and 1 minor criterion, or 1 major and 3 minor criteria, or 5 minor criteria. In recent human guidelines, 1 major and 3 minor criteria, or 5 minor criteria, are considered sufficient evidence for diagnosis of definitive endocarditis.19
Infections of the Cardiovascular System
Etiologic Agents, Epidemiology, and Clinical Features
Term
Definition
Bacteremia
The presence of viable or cultivable bacteria in the bloodstream
Uncomplicated bacteremia
Positive blood culture results and exclusion of endocarditis; no implants; negative follow-up cultures 2-4 days after the initial set; defervescence within 72 hours of initiation of effective treatment; and no evidence of metastatic sites of infection
Complicated bacteremia
Animals with positive blood culture results that do not meet the criteria for uncomplicated bacteremia
Fungemia
The presence of viable fungi in the bloodstream
Systemic inflammatory response syndrome (SIRS)
The clinical manifestation of systemic inflammation due to both noninfectious and infectious etiologies. Criteria for dogs and cats have been adapted from those in human medicine, but these have varied slightly in the cutoff values used. A single definition for dogs or cats has not been widely accepted. SIRS criteria are nonspecific and must be interpreted in light of an underlying disease.
Dogs: 2 of 4 of temperature <100°F or >103.0°F (hypothermia or fever), HR > 140 beats/min, nonpanting RR > 30 breaths/min or PCO2 < 32 mm Hg (venous or arterial), WBC <6000 or >16,000 cells/µL, or >3% band neutrophils
Cats: 3 of 4 of temperature <100°F or >103.5°F, HR < 140 or >225 bpm, RR > 40 breaths/min, WBC > 19,500 or <5000 cells/µL, or >5% band neutrophils
Sepsis
SIRS due to a proven or clinically suspected infection
Severe sepsis
Sepsis that is accompanied by dysfunction of organs remote from the site of infection, or sepsis-induced tissue hypoperfusion
Sepsis-induced tissue hypoperfusion
Sepsis that is accompanied with hypotension, an elevated lactate concentration, or oliguria either before or after adequate fluid loading
Septic shock
Sepsis that is accompanied by hypotension (systolic blood pressure < 90 mm Hg or mean arterial pressure < 70 mm Hg) that does not resolve with fluid resuscitation alone (i.e., vasopressor therapy is required)
Multiple organ dysfunction syndrome (MODS)
Dysfunction of two or more organ systems (distant to site of infection) in an animal with SIRS/sepsis
Infective endocarditis
Infection of one or more endocardial surfaces of the heart; almost always involves a cardiac valve
Bacteremia
Sepsis
Organ
Clinical Abnormalities
Neurologic system
Obtundation, rarely focal signs and seizures; autonomic dysfunction
Heart
Heart rate variability, arrhythmias (ventricular or supraventricular), decreased contractility
Vasculature
Hypotension despite adequate fluid loading
Lungs
Acute lung injury or acute respiratory distress syndrome
Kidneys
Azotemia, oliguria, anuria
Gastrointestinal tract
Impaired barrier function and bacterial translocation, gastrointestinal ulceration, diarrhea, vomiting
Pancreas
Pancreatitis
Endocrine system
Adrenal insufficiency, hypoglycemia
Liver
Cholestatic jaundice
Immune system
Immunosuppression
Coagulation
Disseminated intravascular coagulation with or without hemorrhage
Infective Endocarditis
Microorganism
Prevalence11
Streptococci (primarily S. canis but also S. bovis group organisms)
29%
Bartonella spp.
20%
Staphylococci (S. pseudintermedius, S. aureus, and coagulase-negative staphylococci)
15%
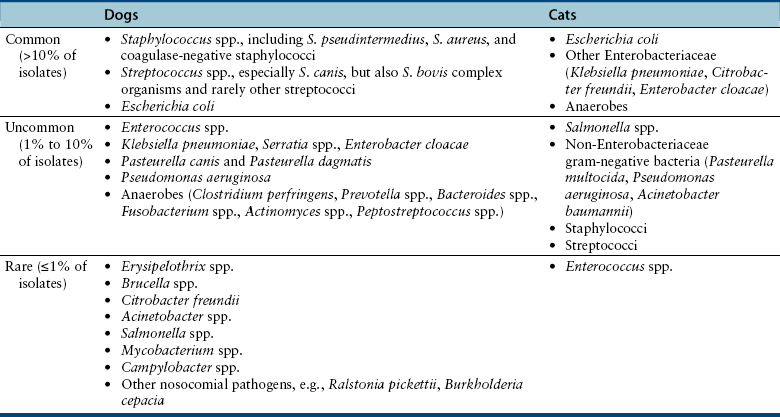
Gram-negative rods (most commonly E. coli, but also Pseudomonas aeruginosa, Salmonella spp., Citrobacter freundii, Klebsiella pneumoniae, Proteus spp., Pasteurella spp., Brucella spp.)
15% (7% E. coli; <5% other species)
Other gram-positive bacteria (Erysipelothrix spp., Granulicatella adiacens, Actinomyces spp., Corynebacterium spp., Mycobacterium spp.)
7% (<5% each species)
Enterococcus spp.
5%
Fungi (e.g., Aspergillus spp.)
<5%
Infectious Myocarditis
Pathogen Type
Examples
Affected Host Species
Viruses
West Nile virus
Canine parvovirus 2 variants
Feline panleukopenia virus
Pseudorabies virus
D
D
C
D
Bacteria
Gram-negative and gram-positive bacteria (e.g., extension of endocarditis)
Bartonella spp.
Borrelia burgdorferi
Rickettsia rickettsii
D, C
D, C
D
D
Fungi
Blastomyces dermatitidis
Candida albicans
Cryptococcus neoformans
Aspergillus spp.
Paecilomyces variotii
D
D
D
D
D
Algae
Prototheca spp.
D
Protozoa
Trypanosoma cruzi
Sarcocystis felis
Leishmania
Neospora caninum
Hepatozoon americanum
Toxoplasma gondii
D
C
D
D
D
D, C
Infectious Pericarditis
Diagnosis
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Veterian Key
Fastest Veterinary Medicine Insight Engine
